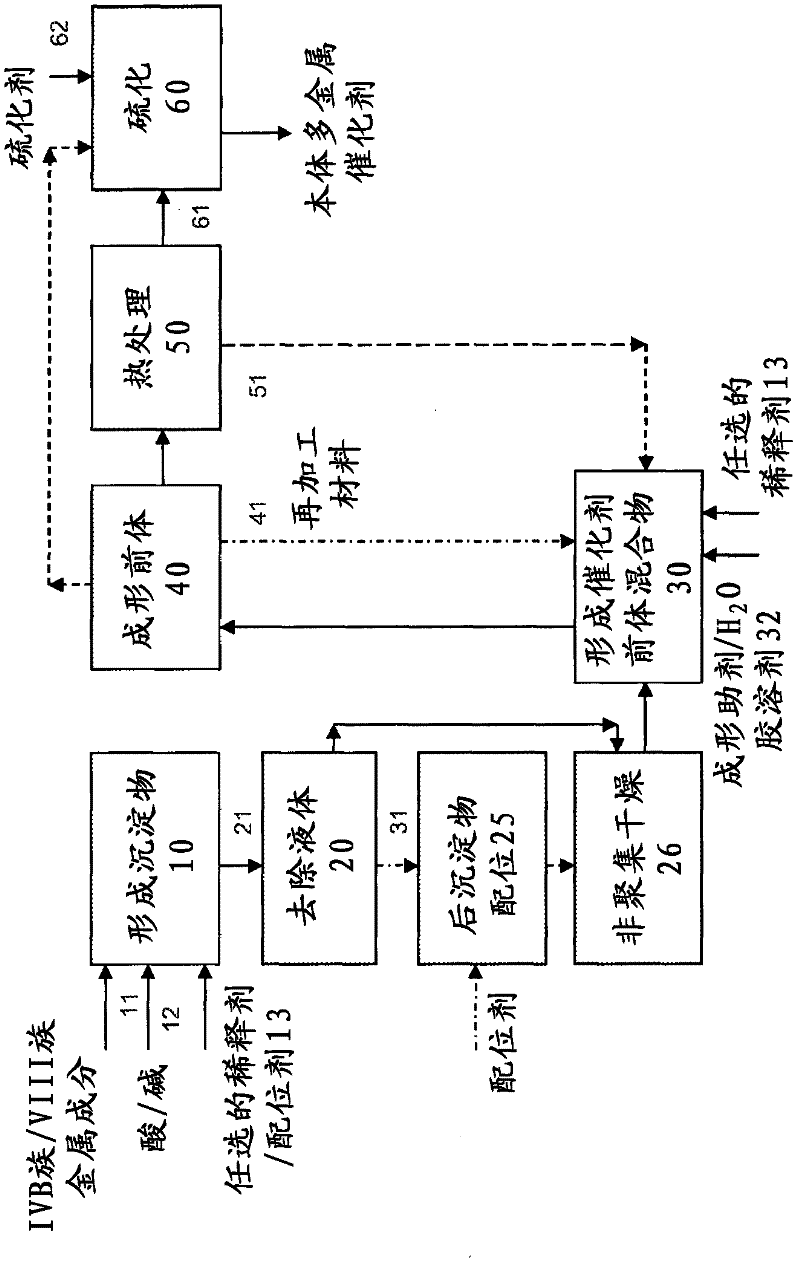
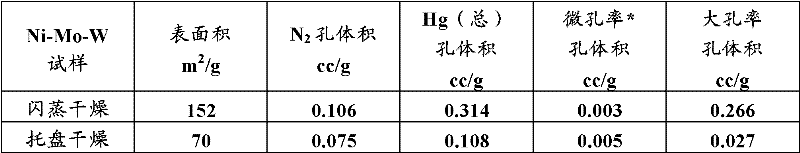
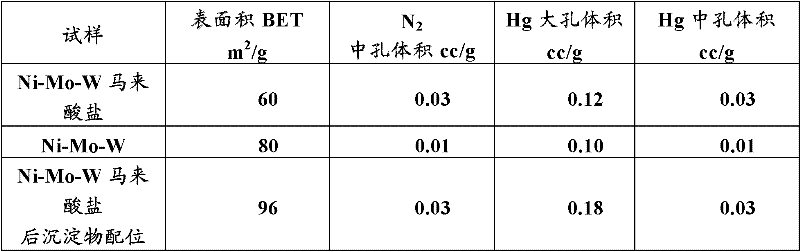
Hydroconversion multi-metallic catalyst and method for making thereof
A multi-metal catalyst, catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of small reactor volume, affect reactor performance, reduce Utilization efficiency and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1 Ni-Mo-W-maleate catalyst precursor
[0084] According to the following preparation formula (NH 4 ){[Ni 2.6 (OH) 2.08 (C 4 h 2 o 4 2- ) 0.06 ](Mo 0.35 W 0.65 o 4 ) 2} catalyst precursor: 52.96 g of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O was dissolved in 2.4L deionized water. The pH of the prepared solution is 2-3. 52.96g of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O is dissolved in the above solution. The pH of the prepared solution is 5-6. Then, 73.98g of ammonium metatungstate powder was added to the above solution and stirred at room temperature until completely dissolved. To this solution was added 90 ml of concentrated (NH 4 ) OH. The resulting molybdate / tungstate solution was stirred for 10 minutes and the pH was monitored. The solution has a pH of 9-10. A second solution was prepared containing 174.65 g of Ni(NO 3 ) 2 ·6H 2 O and the solution was heated to 90 °C. Then, slowly add hot nickel solutio...
Embodiment 2
[0085] Example 2 Ni-Mo-W catalyst precursor
[0086] According to the following preparation formula (NH 4 ){[Ni 2.6 (OH) 2.08 ](Mo 0.35 W 0.65 o 4 ) 2} catalyst precursor: 52.96 g of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O was dissolved in 2.4L deionized water. The pH of the prepared solution is 5-6. Then, 73.98g of ammonium metatungstate powder was added to the above solution and stirred at room temperature until completely dissolved. To the solution was added 90 ml concentrated (NH 4 ) OH. The resulting molybdate / tungstate solution was stirred for 10 minutes and the pH was monitored. The solution has a pH of 9-10. A second solution was prepared containing 174.65 g of Ni(NO 3 ) 2 ·6H 2 O and the solution was heated to 90 °C. Then, slowly add hot nickel solution to the molybdate / tungstate solution over 1 hour. The resulting mixture was heated to 91 °C and stirring was continued for 30 minutes. The pH of the solution is 5-6. A blue-green prec...
Embodiment 3
[0087] Example 3: The precipitate from Example 2 was dispersed in a solution of 10.54 g maleic acid dissolved in 1.8 L deionized water and heated to 70°C. The resulting slurry was stirred at 70°C for 30 minutes, filtered, and the collected precipitate was vacuum dried overnight at room temperature. The material was then further dried at 120°C for 12 hours. The as-prepared material has a typical XRD pattern, in There is a broad peak at , indicating an amorphous Ni-OH-containing material. The resulting material has a BET surface area of 101 m 2 / g, the average pore volume is about 0.12-0.14cc / g, and the average pore diameter is about 5nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com