Strontium cerate-based high-temperature proton conductor material and preparation method and application thereof
A proton conductor and high temperature technology, applied in the field of high temperature proton conductors, can solve the problems of poor chemical stability of high temperature proton conductors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Using high-purity SrCO 3 , CeO 2 , SnO 2 and Y 2 o 3 powder (analytically pure), according to the chemical formula SrCe 0.9-x sn x Y 0.1 o 3-δ The stoichiometric ratio (x is Sn 4+ Doping amount, x=0, 0.1, 0.15, 0.2) carry out weighing batching; 2 The ball was wet milled for 10 hours, dried, passed through a 200-mesh sieve, and roasted for 10 hours at 1250°C in an air atmosphere; 2 Wet mill the ball for 10 hours, add 1% PVB as binder, mix evenly, dry, pass through a 200-mesh sieve, shape, and then press isostatically under 200 MPa to obtain a green body; finally, the green body is roasted at 600°C for 2 After debinding for 1 hour, it was sintered at 1450 °C for 10 hours to obtain SrCe 0.9-x sn x Y 0.1 o 3-δ Material.
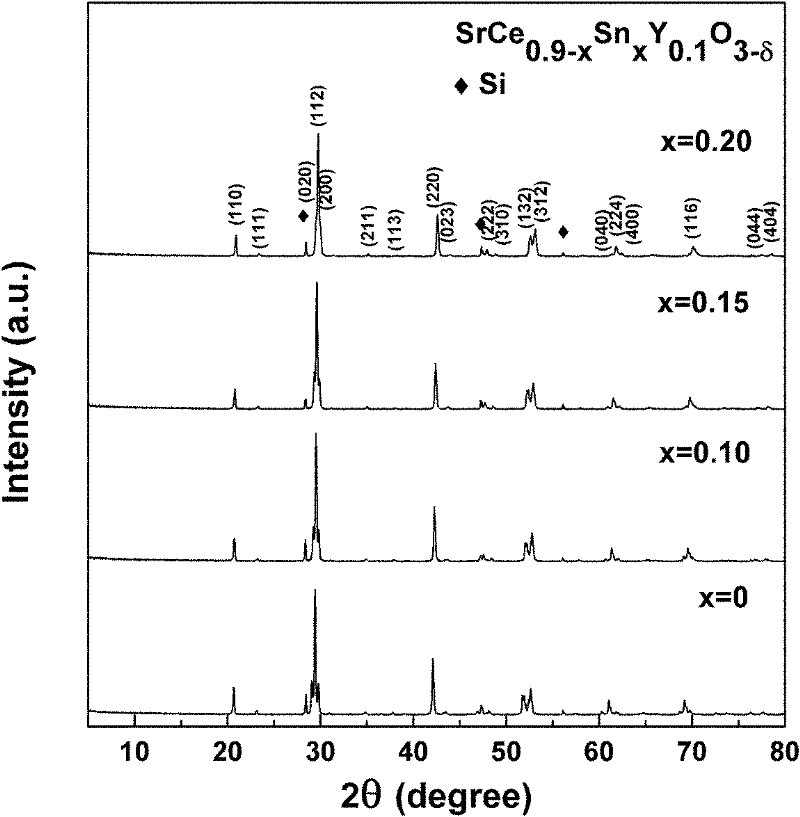
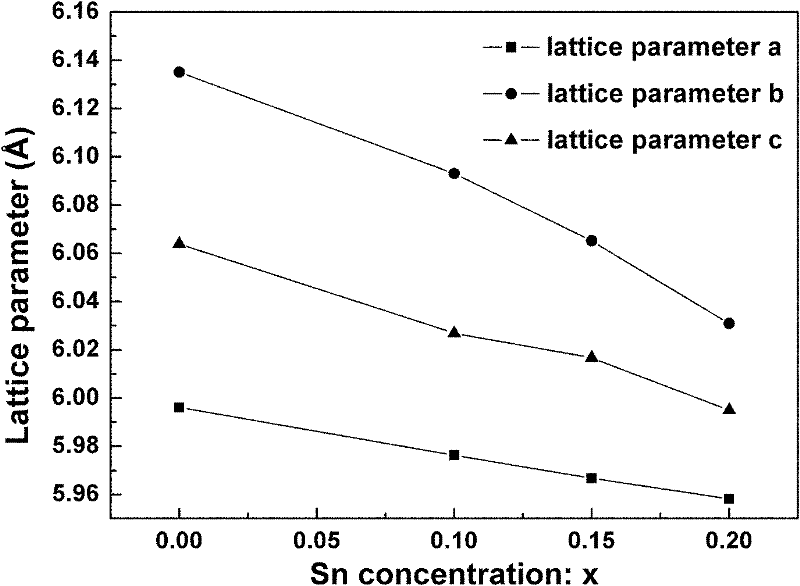
[0043] figure 1 Is the different Sn prepared in this example 4+ doped SrCe 0.9-x sn x Y 0.1 o 3-δ (x=0, 0.1, 0.15, 0.2) XRD spectra of ceramics (corrected by doping high-purity Si to calculate lattice parameters). It can be seen that ...
Embodiment 2
[0047] Using high-purity SrCO 3 , CeO 2 , SnO 2 and Y 2 o 3 powder (analytically pure), according to the chemical formula SrCe 0.9-x sn x Y 0.1 o 3-δ The stoichiometric ratio (x is Sn 4+ Doping amount, x=0, 0.1, 0.15, 0.2) carry out weighing batching; 2 The ball was wet milled for 8 hours, dried, passed through a 200-mesh sieve, and roasted for 10 hours at 1300°C in an air atmosphere; 2 Wet mill the ball for 10 hours, add 1.5% PVB as binder, mix evenly, dry, pass through a 200-mesh sieve, shape, and then isostatically press under 250MPa pressure to obtain a green body; finally, the green body is roasted at 600°C for 2 After debonding for 1 hour, sintering at 1500°C for 10 hours yields SrCe 0.9-x sn x Y 0.1 o 3-δ Material.
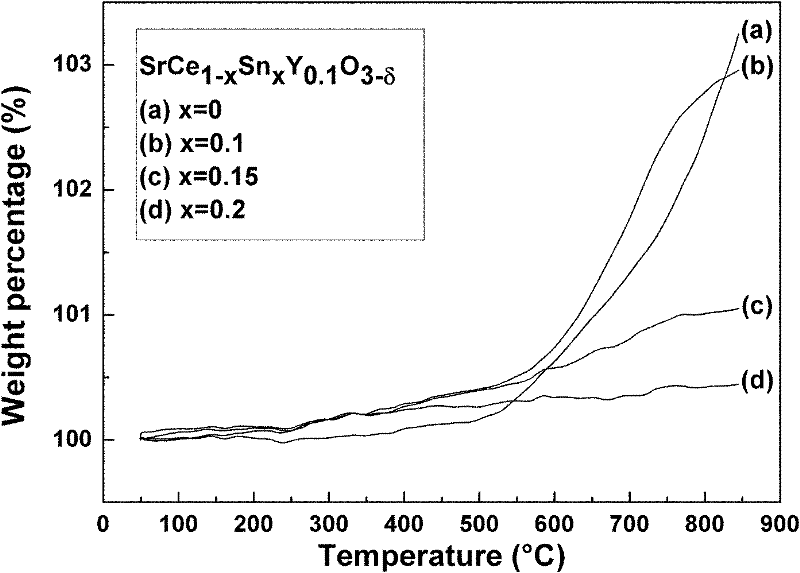
[0048] Figure 5 Different Sn prepared for this example 4+ doped SrCe 0.9-x sn x Y 0.1 o 3-δ (x=0, 0.1, 0.15, 0.2) XRD spectra of ceramics before and after boiling in boiling water for 4 hours. It can be seen that the undoped Sn 4+ Aft...
Embodiment 3
[0053] Using high-purity SrCO 3 , CeO 2 , SnO 2 and Y 2 o 3 powder (analytically pure), according to the chemical formula SrCe 0.9-x sn x Y 0.1 o 3-δ The stoichiometric ratio (x is Sn 4+ Doping amount, x=0, 0.1, 0.15, 0.2) carry out weighing batching; 2 The ball was wet milled for 10 hours, dried, passed through a 200-mesh sieve, and calcined for 10 hours at 1200°C in an air atmosphere; 2 Ball wet grinding for 10 hours, adding 1% PVB as binder, mixing evenly, drying, passing through 200 mesh sieve, forming, and then isostatic pressing under 150MPa pressure to obtain green body; finally, the green body was roasted at 600°C for 2 After removing the binder for 1 hour, it was divided into six parts and sintered at 1250, 1300, 1350, 1400, 1450, and 1500 ° C for 10 hours to obtain SrCe at different sintering temperatures. 0.9-x sn x Y 0.1 o 3-δ Material.
[0054] Figure 6 Different Sn prepared for this embodiment 4+ doped SrCe 0.9-x sn x Y 0.1 o 3-δ (x=0, 0.1, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com