Oral liquid preparation
A liquid preparation and liquid technology, applied in the field of medical liquid preparations, can solve the problems of improving dissolution, undisclosed, blonanserin solubility, and dissolution have not been studied, and achieve good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
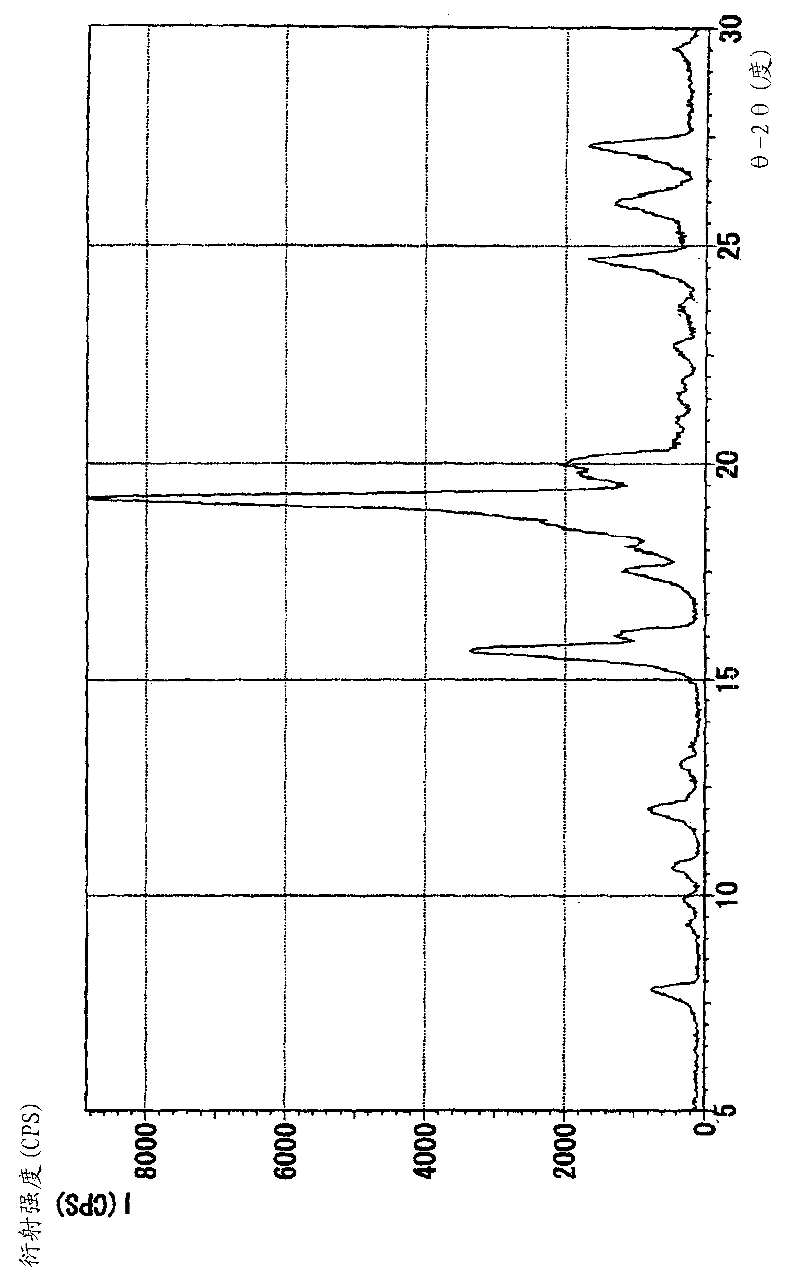
Image
Examples
Embodiment 1~4
[0059] Citric acid (anhydride) was added to purified water to prepare a 25 mM citric acid aqueous solution. Then, according to the formula of Example 1 in Table 1, blonanserin was added to the citric acid aqueous solution, and a magnetic stirrer was used to stir at room temperature until the blonanserin was dissolved to prepare a liquid preparation containing blonanserin (implementation example 1). After preparation, the pH of the liquid formulation is measured.
[0060] Similarly, according to the method of Example 1, each component was charged in the amount described in Table 1, and liquid preparations containing blonanserin were respectively prepared (Examples 2 to 4).
[0061] [Table 1]
[0062] .
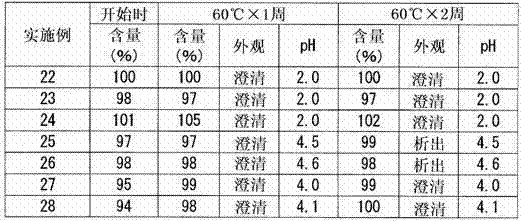
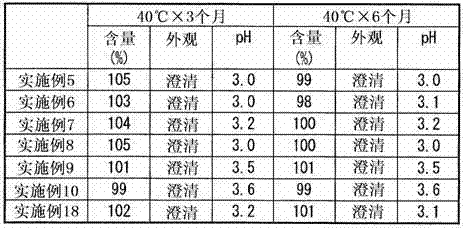
Embodiment 5~10
[0064] A 25 mM citric acid aqueous solution was prepared in the same manner as in Example 1. Next, each component was added in the amount described in Table 2 to this citric acid aqueous solution, and the liquid preparation containing blonanserin was prepared by the method similar to Example 1 (Examples 5-10).
[0065] [Table 2]
[0066] .
Embodiment 11~13
[0068] Using purified water and malic acid, a 50 mM malic acid aqueous solution was prepared. Next, each component was added to the malic acid aqueous solution in the amount described in Table 3, and a liquid preparation containing blonanserin was prepared by the same method as in Example 1 (Examples 11 to 13).
[0069] [table 3]
[0070] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com