Process for producing polyhydric phenol
A technology of polyphenols and group elements, applied in the field of preparation of polyphenols, can solve problems such as DHQ yields that are not specified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach
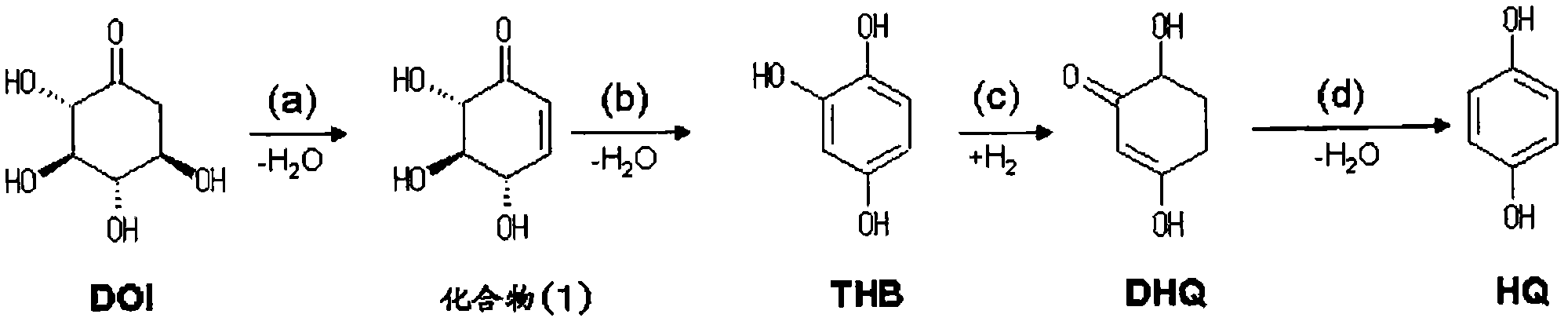
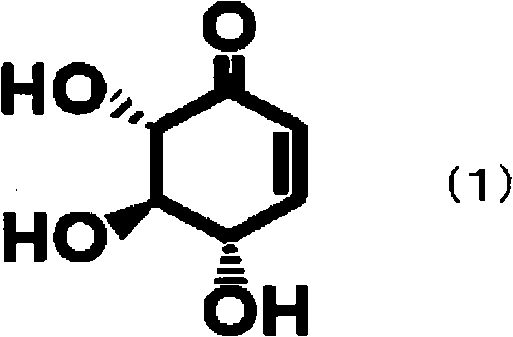
[0027] In this embodiment, the steps (a) and (b) of the present invention will be specifically described. That is, in the present embodiment, the second step (b) of the present invention is carried out by dehydration reaction in the absence of an acid catalyst. According to the above method, in the reaction to obtain THB from DOI, the compound represented by the above formula (1) is used as a reaction intermediate, and the dehydration reaction is carried out in the absence of an acid catalyst. Accordingly, the amount of by-products generated in the presence of the acid catalyst can be reduced. Therefore, THB can be produced industrially using chemical methods.
[0028] In the method of this embodiment, it is preferable to dissolve the compound represented by said formula (1) in a reaction solvent, and to perform dehydration reaction, heating to 120 degreeC or more. The reaction temperature is more preferably 120°C to 200°C. Water can be used as a reaction solvent. The conc...
no. 2 Embodiment approach
[0040] In this embodiment, the 3rd step (c) and 4th step (d) of this invention are demonstrated concretely.
[0041] As described above, as a method for industrially producing hydroquinone using a chemical method, the prior art described in the above literature still has room for improvement in terms of yield. For example, in the step of synthesizing hydroquinone from THB, further improvement in yield can be expected.
[0042] Non-Patent Document 1 does not disclose DHQ as an intermediate in the step of producing hydroquinone from THB. In addition, the yield of the reaction to obtain DHQ from THB and the yield of the reaction to obtain hydroquinone from DHQ are not specifically described, respectively.
[0043] In addition, considering the purity, the yield of DHQ in Non-Patent Document 2 is 63%. However, in order to replace the existing petroleum method with a chemical method, further improvement in yield is expected.
[0044]In addition, Non-Patent Document 3 does not spe...
no. 3 Embodiment approach
[0093] This embodiment is a method for preparing hydroquinone using DOI as a starting material. Said method implements the steps represented by (e) and (f) in addition to the steps (a) to (d) of the present invention. Specifically, as described below, it is performed in the order of (a-1), (b-1), (c-2), (d-2), (e), and (f).
[0094]Step (a-1): In the absence of an acid catalyst, the compound represented by the above formula (1), ie (4S, 5R, 6S)-4,5,6-trihydroxy-2-ring, is prepared from DOI by dehydration reaction Hexen-1-one.
[0095] Step (b-1): THB is produced from the compound represented by the above formula (1) obtained in the step represented by (a-1) by dehydration reaction in the absence of an acid catalyst.
[0096] Step (c-2): The alkali metal of DHQ is prepared from THB obtained in the step represented by (b) by a catalytic hydrogenation reaction using a metal catalyst containing an iron group element as a metal component in the presence of a hydroxide of an alkal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com