Method for preparing carboxyl anhydride
A manufacturing method and technology of carboxylic anhydrides, which are applied in the field of manufacturing carboxylic anhydrides, can solve problems such as loss of rhodium catalysts, achieve the effects of reducing insoluble tars, expanding the range of reaction operations, and improving space-time yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
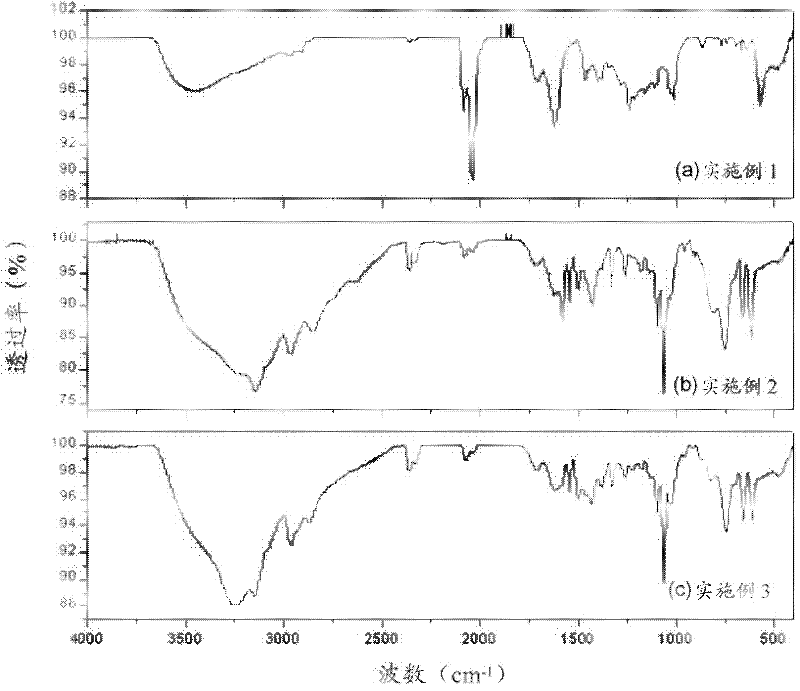
[0025] 0.29g of ethylene diacetate, 0.37g of IrCl 3 ·XH 2 O (wherein Ir atoms account for 52.5%) and 100 ml of ethanol were added into the reaction flask, heated to 100 degrees Celsius and then refluxed for 20 hours. After cooling to normal temperature, filter to obtain a black precipitated solid and a yellow-brown solution. Concentrate the yellow-brown solution and wash it with ether, then dry it to remove the solvent, and then measure the infrared spectrum of the product after the reaction ( figure 1 In (a)), and as a control experiment.
Embodiment 2
[0027] 0.22g of N-acetylimidazole, 0.37g of IrCl 3 ·XH 2 O (wherein Ir atoms account for 52.5%) and 100 ml of ethanol were added into the reaction flask, heated to 100 degrees Celsius and then refluxed for 20 hours. After being cooled to normal temperature, filter to obtain a yellow solid and a yellow solution. The yellow solution is concentrated and rinsed with ether, then dried to remove the solvent, and the infrared spectrum of the product after the reaction can be determined ( figure 1 In (b)), and as a control experiment.
Embodiment 3
[0029] 0.29g of ethylene diacetate, 0.22g of N-acetylimidazole, 0.37g of IrCl 3 ·XH 2 O (wherein Ir atoms account for 52.5%) and 100 ml of ethanol were added into the reaction flask, heated to 100 degrees Celsius and then refluxed for 20 hours. After being cooled to normal temperature, filter to obtain a yellow solid and a yellow solution. The yellow solution is concentrated and rinsed with ether, then dried to remove the solvent, and the infrared spectrum of the product after the reaction can be determined ( figure 1 in (c)).
[0030] By comparing the infrared spectrograms of embodiment 3 and embodiment 1 and embodiment 2, it can be clearly seen that the spectrogram signal of embodiment 3 is similar to the spectrogram signal of embodiment 2, that is, the N-acetyl imidazole and iridium ion Coordinating power is better. It shows that the addition of such protective agent in the present invention does have the effect of preventing the interaction between ethylene diacetate an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com