Adjuvant composition comprising (poly-gamma-glutamate)-chitosan nanoparticles
A technology of chitosan nanometer and glutamic acid, which can be used in drug combinations, medical preparations containing active ingredients, microorganisms, etc., can solve the problem of not yet inducing immune response and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of ultra-high molecular weight poly-γ-glutamic acid
[0060] Prepared as a basal medium for the production of poly-γ-glutamic acid (supplemented with 3% L-glutamic acid; containing 3% glucose, (NH 4 ) 2 SO 4 1%, KH 2 PO 4 0.27%, Na 2 HPO 4 12H 2 O 0.17%, NaCl 0.1%, Sodium Citrate 0.5%, Soybean Peptone 0.02%, MgSO 4 ·7H 2 O 0.7%, vitamin solution 10ml / L, PH 6.8) and sterilized. Inoculate the medium (LB medium) of Bacillus subtilis var.chungkookjang (Bacillus subtilis var.chungkookjang) (KCTC 0697BP) into the medium of a 5L fermenter (working volume 3L) with a concentration of 4%, and stir with 500rpm, 1vvm Aeration rate and 37°C fermentation for 48 hours. Bacterial cells were then removed using a small filter press (1% diatomaceous earth), and the remaining material was used as a sample solution containing poly-γ-glutamic acid.
[0061] The pH of the sample solution containing poly-γ-glutamic acid was adjusted to 2.0 with a 2N sulfu...
Embodiment 2
[0062] Embodiment 2: Preparation of poly-γ-glutamic acid-chitosan nanoparticles
[0063] Nanoparticles as an adjuvant were prepared using poly-γ-glutamic acid and chitosan (Amicogen Co., Ltd., Korea) prepared in Example 1.
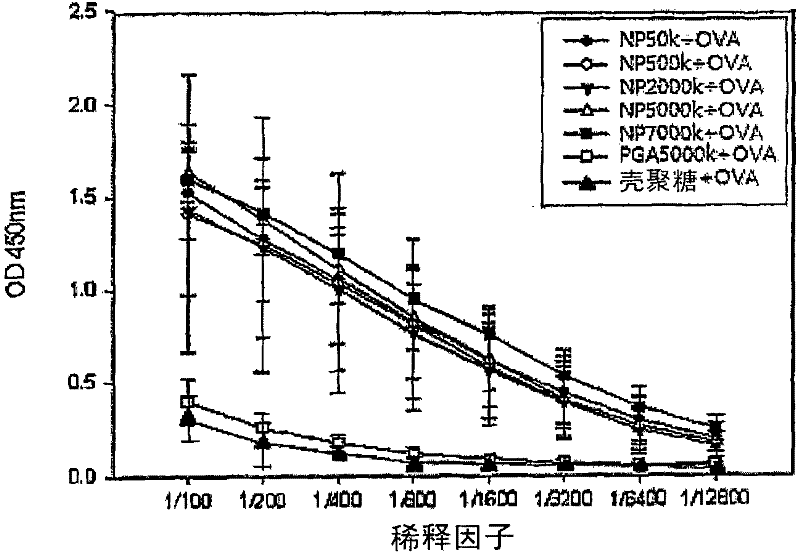
[0064]Specifically, poly-γ-glutamic acid and chitosan were dissolved in 0.85% NaCl solution. The poly-gamma-glutamic acid solution and the chitosan solution are mixed with each other at a ratio of 1:1-8:1 (poly-gamma-glutamic acid:chitosan), thereby preparing the surface negatively charged poly-gamma-glutamic acid Acid-chitosan nanoparticles. The particle size and surface charge of the prepared nanoparticles were measured using DLS (Dynamic Light Scattering). As a result, it can be seen that the prepared nanoparticles have a particle size of 200-300 nm and a surface charge of -20.8 mV. In addition, the surface morphology of the as-prepared nanoparticles was observed with an electron microscope (see figure 1 ).
[0065] [Table 1] Particle size and surf...
Embodiment 3
[0067] Example 3: Preparation of Poly-γ-Glutamate-Chitosan Nanoparticles Using Multiple Sequences of Addition of Target Proteins
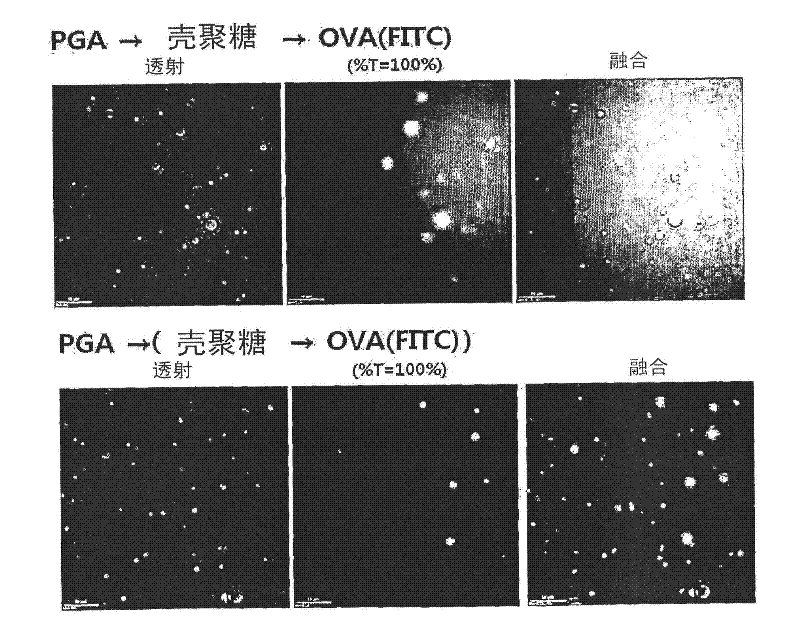
[0068] In order to verify that the poly-γ-glutamic acid-chitosan nanoparticles prepared in Example 2 are used as an adjuvant to enhance the function of producing antibodies to the corresponding protein, the pI value of the corresponding protein is determined, and a variety of the protein is used Sequence of additions to prepare nanoparticles. First, OVA-FITC having a pI value of 5.2 obtained by bonding a fluorescent substance FITC to OVA protein (Sigma Corporation, USA) was bonded to polyγ-glutamic acid-nanoparticles. Specifically, the following two kinds of nanoparticles were prepared: those prepared by mixing poly-γ-glutamic acid and OVA-FITC, and then adding chitosan thereto, and those prepared by mixing chitosan and OVA-FITC, and then adding Nanoparticles prepared by adding poly-γ-glutamic acid. The degree of bonding of OVA in the prepared na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com