Chitosan nano drug carrier with pH and temperature response and preparation method and application thereof
A nano-drug carrier, chitosan nano technology, applied in the direction of drug combination, pharmaceutical formula, non-active ingredient medical preparations, etc., can solve the problem of external signal stimulation, reduce toxic side effects, mild conditions, and improve bioavailability degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of solid cyclodextrinized chitosan nanoparticles with pH / temperature dual response
[0034] (1) Synthesis of cyclodextrinized chitosan (CS-g-CD): Weigh 2g of chitosan (deacetylation degree 95%, viscosity average molecular weight 100,000) and dissolve it in 100mL 1% acetic acid aqueous solution (pH~ 5.6) to obtain a chitosan solution with a concentration of 2% (w / v); weigh 2g of carboxymethylated cyclodextrin and dissolve it in 50mL of deionized water to obtain a cyclodextrin with a concentration of 4% (w / v) solution; add 2g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 1g N-hydroxysuccinimide (NHS ), stirring and reacting at room temperature for 2 h to activate the carboxyl group of the cyclodextrin derivative to obtain an activated cyclodextrin solution. The activated cyclodextrin solution is fully mixed with the chitosan solution, stirred and reacted at room temperature for 24 hours, the cyclodextrin carboxylated derivative is modifi...
Embodiment 2
[0038] Preparation of Hollow Cyclodextrinized Chitosan Nanoparticles Responsive to pH / Temperature
[0039] Based on Example 1, the difference is: modify the preparation process of the TPP solution described in step (2) of Example 1 to: dissolve 8 mg of TPP in 20 mL of deionized water to obtain a 0.4 mg / mL TPP solution, and the rest Steps are all the same as in Example 1.
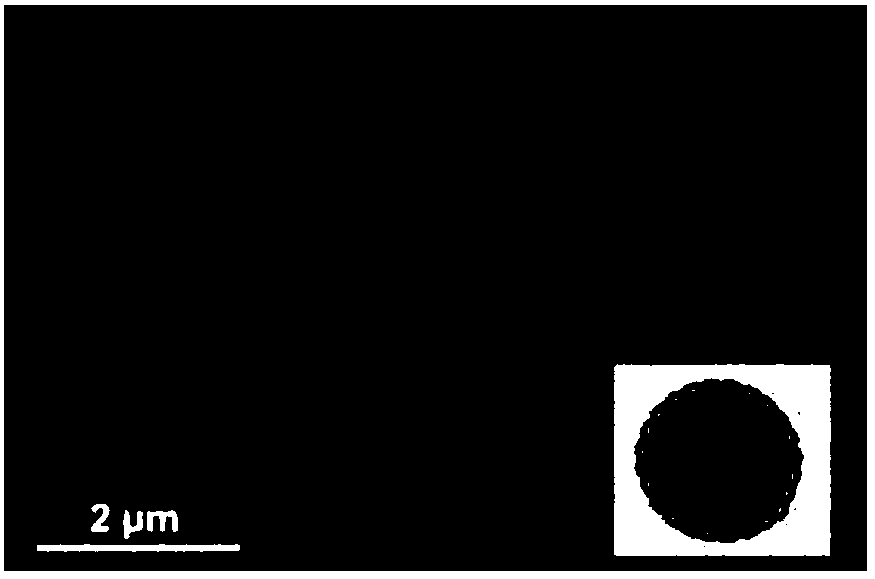
[0040] The cyclodextrinized chitosan nanoparticles prepared in this example are as follows: figure 2 As shown, it is a hollow spherical particle with an average diameter of about 250nm, wherein the chitosan modified by TPP cross-linking forms a hollow hydrophobic core, and the hydrophilic cyclodextrin groups are closely arranged side by side to form a shell. Compared with the solid cyclodextrinized chitosan nanoparticles prepared in Example 1, the hydrophobic core of the nanoparticles prepared in this example is a hollow structure, which may be because the ions of TPP and chitosan in this example The reas...
Embodiment 3
[0042] Preparation of cyclodextrinized chitosan nanoparticles loaded with doxorubicin and pH / temperature dual response
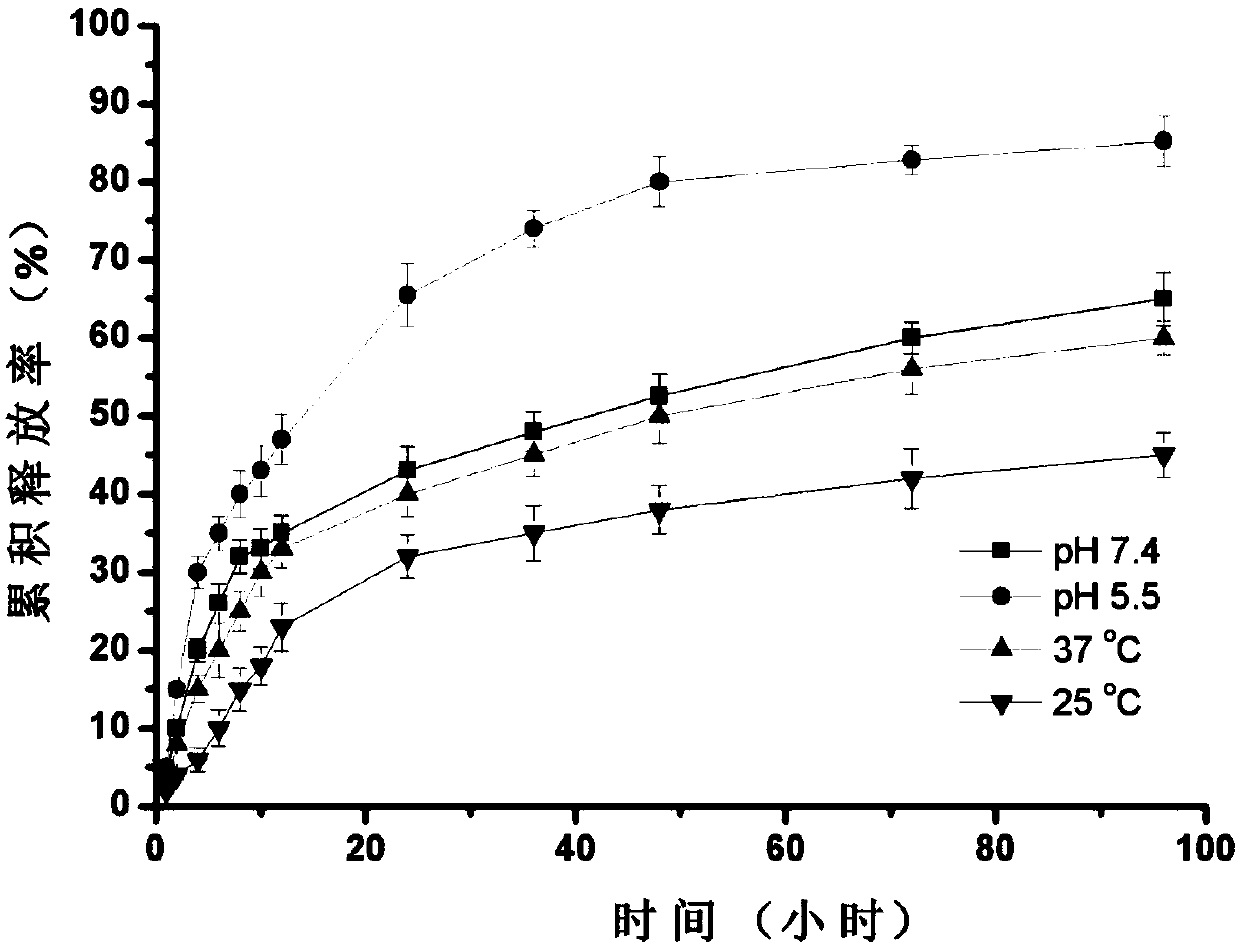
[0043] Dissolve 1 mg of doxorubicin hydrochloride in 5 mL of N,N-dimethyloxalamide (DMF), add 2 μL of triethylamine to desalt the doxorubicin hydrochloride to obtain a solution with a concentration of 0.2 mg / mL of doxorubicin, and dissolve the The drug solution was stirred and mixed with the cyclodextrinized chitosan solution (20 mL, 0.4 mg / mL) described in step (2) in Example 1 for 2 hours to obtain a cyclodextrinized chitosan solution containing doxorubicin. Adopt the preparation method of the cyclodextrinized chitosan nanoparticle described in embodiment 1 step (2), add TPP solution, stirring reaction, filter respectively in the described cyclodextrinized chitosan solution containing doxorubicin , that is, cyclodextrinized chitosan nanoparticles loaded with doxorubicin and pH / temperature dual response are obtained, and the drug is loaded in the cavity of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com