Method of detecting pneumocandin compounds
A technology of echinocandin and echinocandin, applied in the field of detection of echinocandin compounds, can solve the problems of low echinocandin solubility, limited usefulness for analytical purposes, and low stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
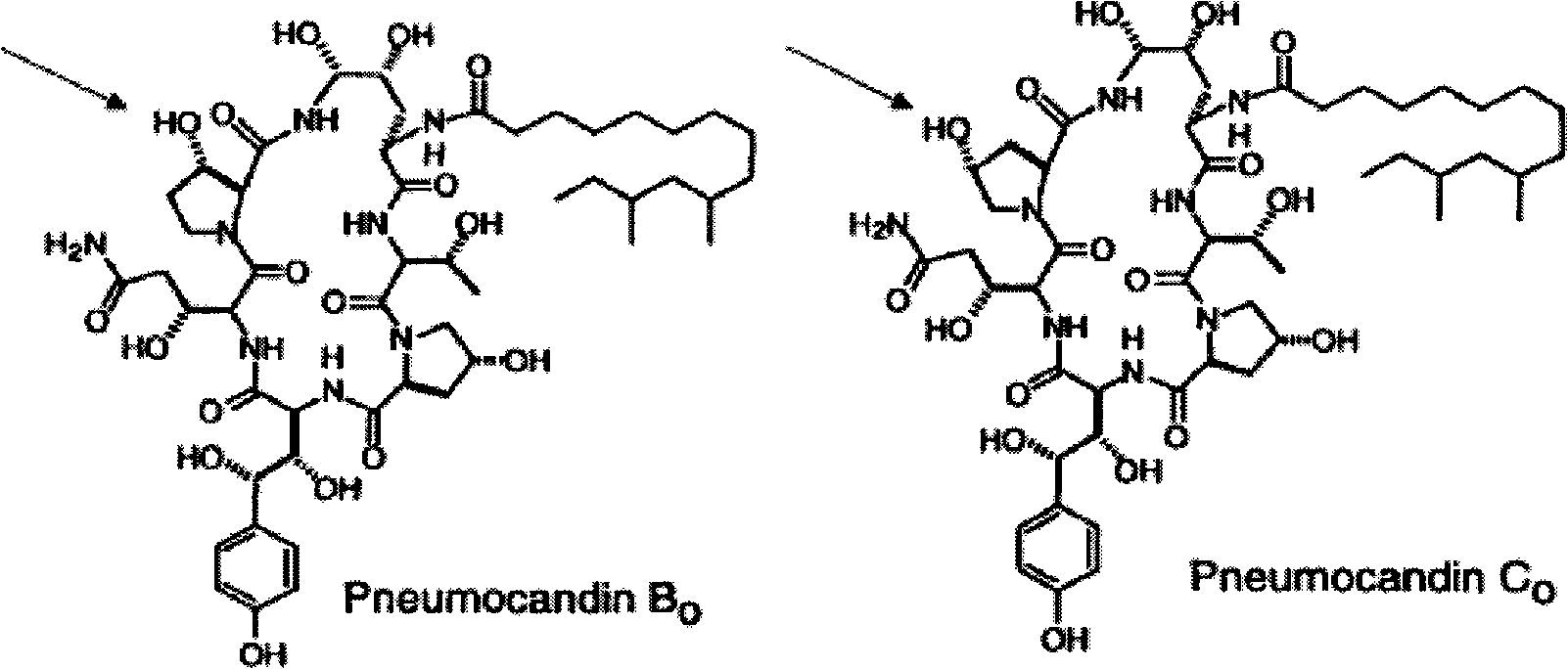
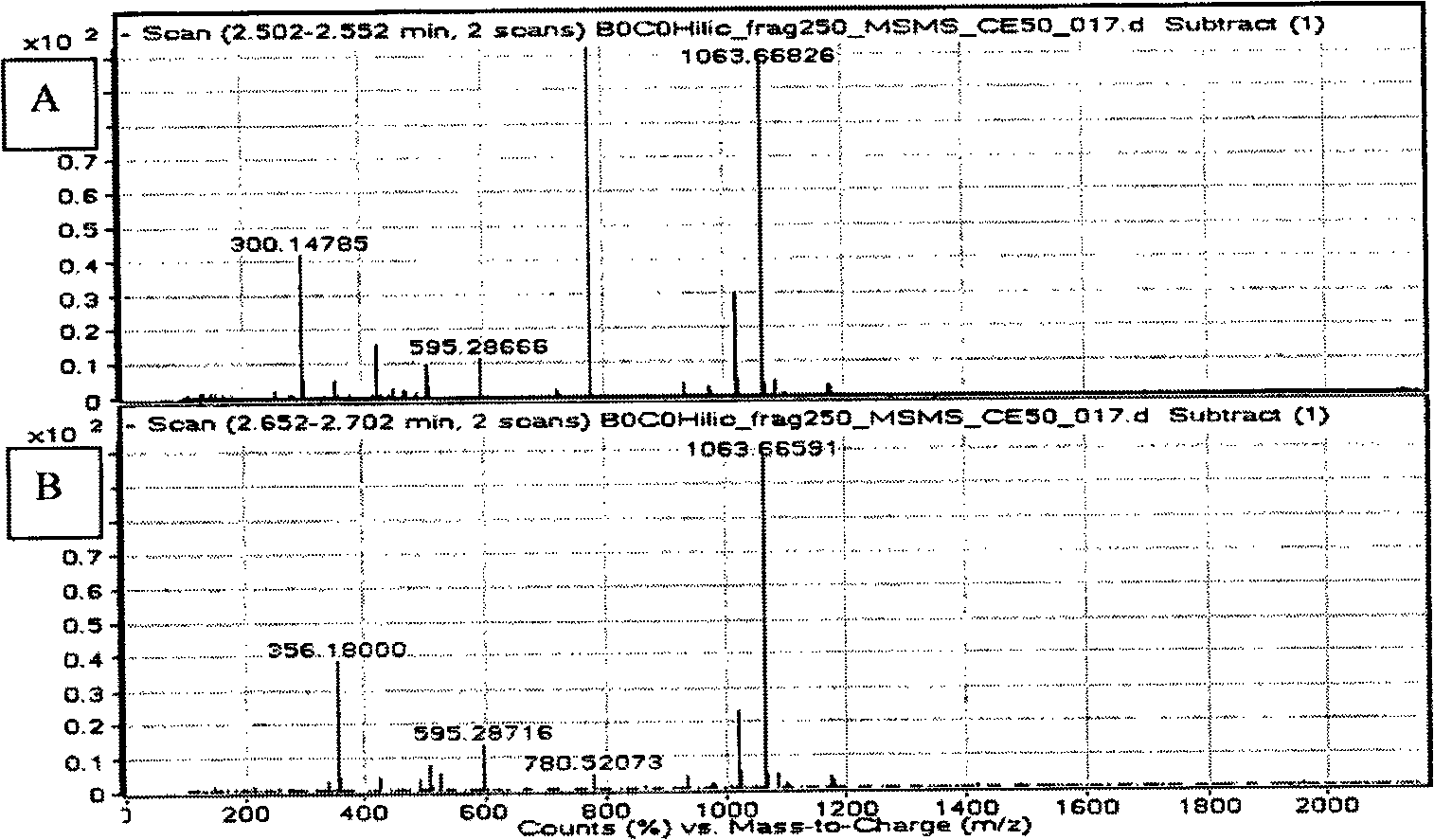
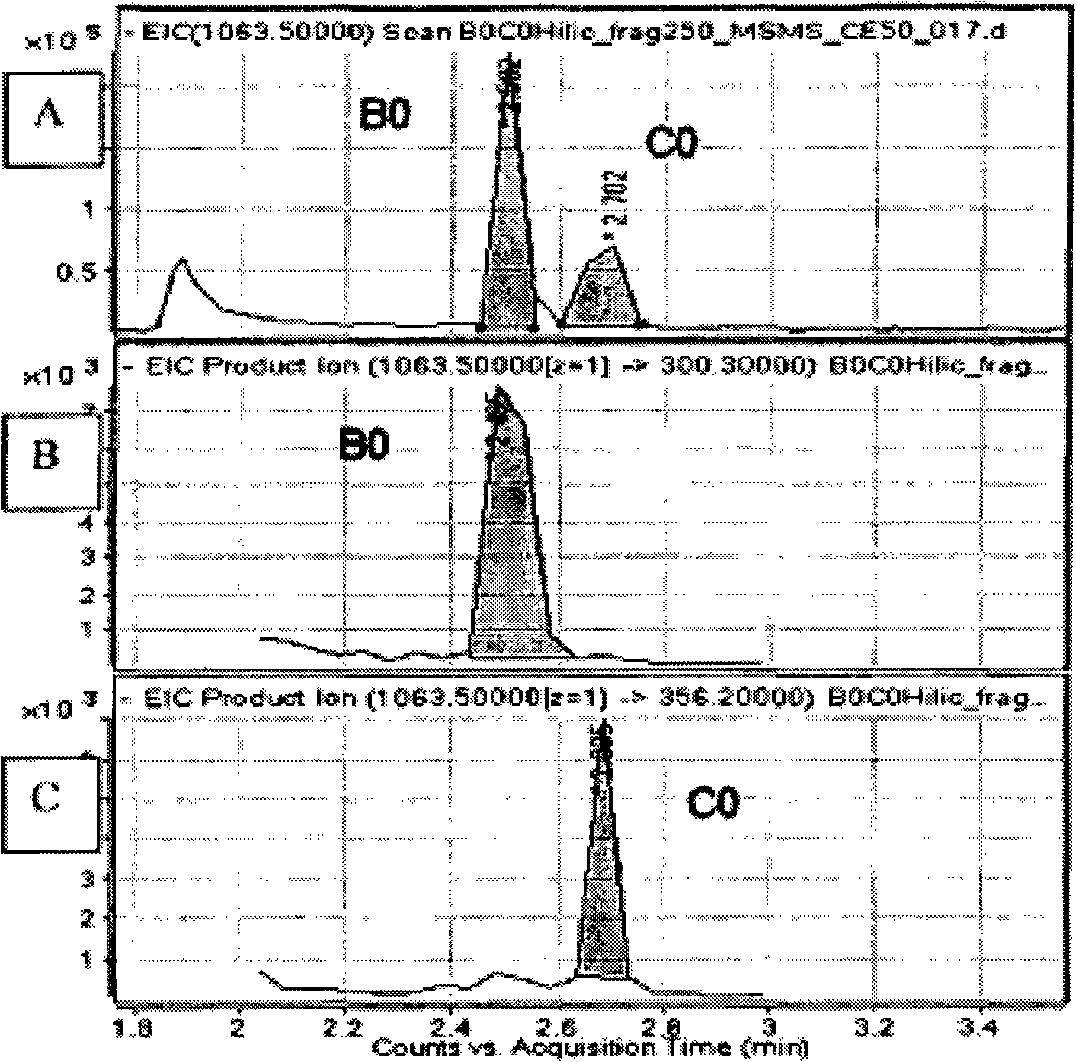
[0046] In this experiment, an Agilent 1200 HPLC system coupled to an Agilent 6520 quadrupole time-of-flight (Q-TOF) mass spectrometer was used. The Agilent 1200HPLC system is composed of a binary pump, a degasser, a constant temperature autosampler and a constant temperature column chamber (the temperature is set at 25°C). A Supelco Ascentis Express Hydrophilic Interaction Chromatography (HILIC) 15 cm x 4.6 mm, 2.7 micron column was used. The mobile phase consisted of 15% v / v 0.1% w / w ammonium acetate (pH=4.5) and 85% v / v acetonitrile. The flow rate was 1 ml / min. The parameters of the MS ion source are as follows: the nebulizer pressure is 50 psig, the drying gas flow rate is 10 L / min, the drying gas temperature is 350° C., and the capillary outlet voltage is 250 volts. LC-MS / MS analysis was performed in negative ion mode with deprotonation. Separation of echinocandin B at m / z 1063 in quadrupole (Q) 0 or Echinocandin C 0 . The separated pseudomolecular ions were then fra...
Embodiment II
[0054] In this experiment, a Thermo Fisher Surveyor HPLC system coupled to a Thermo Fisher LXQ linear ion trap mass spectrometer was used. The Surveyor HPLC system is composed of a quaternary pump, a degasser, a constant temperature autosampler and a constant temperature column chamber (the temperature is set at 40°C). A Supelco Ascentis Si Hydrophilic Interaction Chromatography 15 cm x 2.1 mm, 5 micron column was used. The mobile phase consisted of 13% v / v 0.1% w / w ammonium acetate (pH=4.5) and 87% v / v acetonitrile. The flow rate was 0.2 ml / min. The parameters of the MS ion source are as follows: sheath gas 35 (arbitrary units), auxiliary gas 15 (arbitrary units), capillary column temperature 350 °C, spray voltage 5 kV, LC-MS / MS in negative ion mode and deprotonation change.
[0055] echinocandin B 0 or Echinocandin C 0 was isolated (at m / z 1063) and fragmented in the ion trap (with a collision energy of 13). The ion trap was set to scan between m / z 290 to 1100 (Figures...
Embodiment III
[0063]In this experiment, an Agilent 1200 HPLC system coupled to an Agilent 6410 triple quadrupole (QQQ) mass spectrometer was used. The Agilent 1200HPLC system is composed of a binary pump, a degasser, a constant temperature autosampler, and a constant temperature column chamber (the temperature is set at 25°C). A Supelco Ascentis Express Hydrophilic Interaction Chromatography 15 cm x 4.6 mm, 2.7 micron column was used. The mobile phase consisted of 15% v / v 0.1% w / w ammonium acetate (pH=4.5) and 85% v / v acetonitrile. The flow rate was 1 ml / min. The parameters of the MS ion source are as follows: the nebulizer pressure is 50 psig, the drying gas flow rate is 10 L / min, the drying gas temperature is 325° C., and the capillary outlet voltage is 4000 volts. LC-MS / MS was performed in negative ion mode and deprotonation was performed. Separation of echinocandin B at m / z 1063 in the first quadrupole (Q) 0 or Echinocandin C 0 . The separated pseudomolecular ions are then fragmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com