Adenosine cyclophosphate pharmaceutical composition and preparation method thereof
A cycloadenosine phosphate and cycloadenosine-containing technology are applied in the field of cycloadenosine phosphate pharmaceutical compositions and their preparation, and can solve problems such as poor stability of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
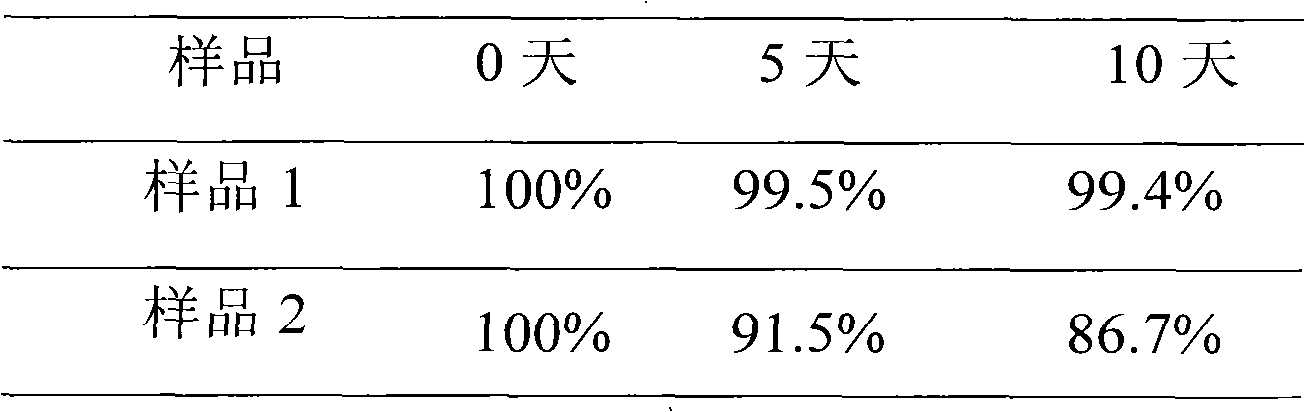
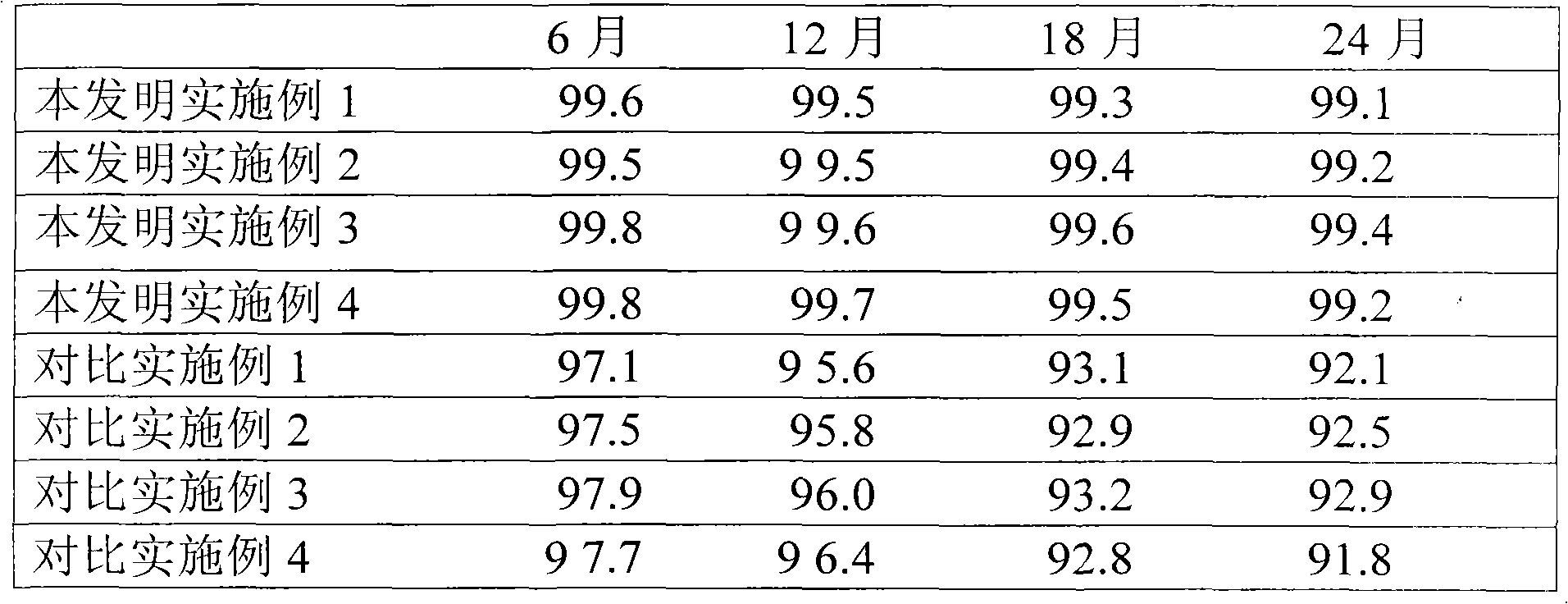
Examples
Embodiment 1
[0054] Example 1 Injection
[0055] Cyclic adenosine monophosphate 20g,
[0057] PEG400 0.5g,
[0058] Appropriate amount of pH regulator,
[0059] Add water for injection to 2000ml;
[0060] Prepared into 1000 sticks.
[0061] The preparation method comprises the following steps:
[0062]1) Weigh the raw and auxiliary materials of the prescription amount;
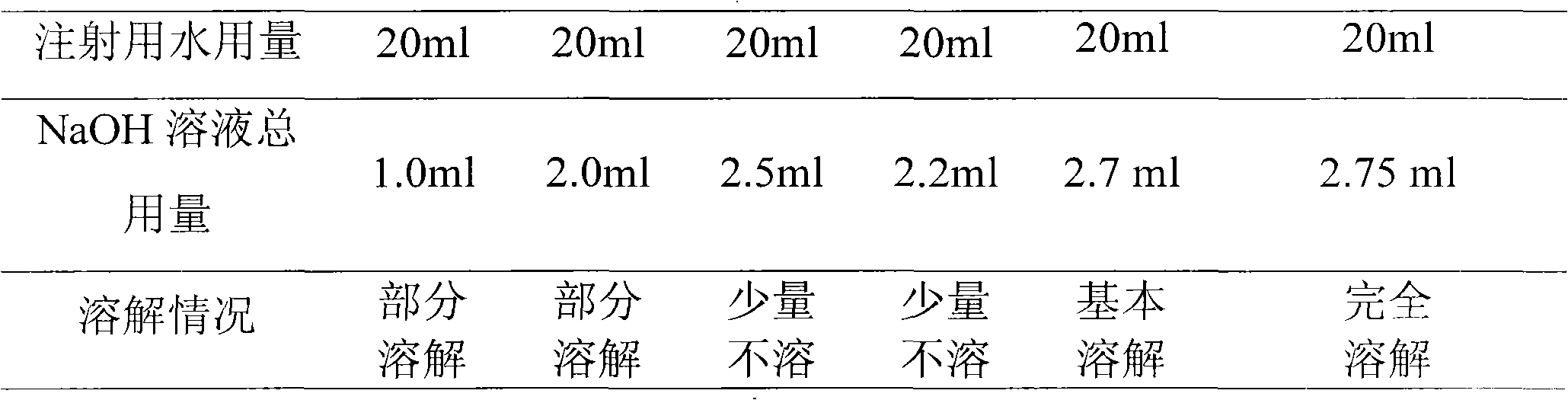
[0063] 2) Sodium hydroxide is added with 70% water for injection, stirred to dissolve, then added cyclic adenosine monophosphate, stirred to dissolve completely;
[0064] 3) Add water for injection to the full amount, stir to mix evenly;
[0065] 4) Measure the pH value of the medicinal solution and adjust the pH value to 5.0-7.0;
[0066] 5) Add activated carbon with a dosing volume of 0.1%, heat and stir for 15 minutes, filter and decarbonize;
[0067] 6) 0.45 micron filter membrane fine filtration, check the clarity;
[0068] 7) Inspection of semi-finished products;
[0069] 8)...
Embodiment 2
[0073] Example 2 Injection
[0074] Cyclic adenosine monophosphate 40g,
[0075] Sodium hydroxide 4.6g,
[0076] PEG400 1g,
[0077] Appropriate amount of pH regulator,
[0078] Add water for injection to 5000ml;
[0079] Prepared into 1000 sticks.
[0080] The preparation method refers to the method of Example 1.
Embodiment 3
[0081] Embodiment 3 freeze-dried powder injection
[0082] Cyclic adenosine monophosphate 20g,
[0083] Sodium hydroxide 2.2g,
[0084] PEG400 0.5g,
[0085] Mannitol 80g,
[0086] Appropriate amount of pH regulator,
[0087] Add water for injection to 1000ml;
[0088] Prepared into 1000 vials.
[0089] The preparation method comprises the following steps:
[0090] 1) Weigh the raw and auxiliary materials of the prescription amount;
[0091] 2) Cyclic adenosine monophosphate is added with 80% sterile water for injection, then sodium hydroxide is added, stirred to dissolve, and then mannitol in the prescribed amount is added, stirred to dissolve;
[0092] 3) Add sterile water for injection to the full amount, stir to mix evenly;
[0093] 4) Measure the pH value of the solution and adjust the pH value to 5.0-7.0;
[0094] 5) Add activated carbon for needles with a dosing volume of 0.05%, heat and stir for 15 minutes, filter and decarbonize;
[0095] 6) 0.22 micron micr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com