Catalyst for preparing hydrogen gas without COx by ammonia decomposition and preparation method thereof
A catalyst and ammonia decomposition technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve problems such as poor stability, high equipment performance, and difficult operation , to achieve the effect of large industrial application significance and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh 2 g of carbon nanotubes (CNTS, Shenzhen Nanoport Co., Ltd., the same below) and place them in a round bottom flask, add 90 ml of concentrated nitric acid (concentrated nitric acid of analytical purity, the same below), and place in a 120°C oil bath Heat to reflux for 4 hours, then filter and wash with water until the filtrate is neutral, and dry at 100° C. for 6 hours to obtain activated carbon nanotubes.
[0026] (2) Determination of saturated water absorption of carbon nanotubes after activation: take 22.4565 g of the mass of an empty beaker, and weigh 1.0015 g of activated carbon nanotubes therein, then the total mass of the empty beaker and non-immersed carbon nanotubes 23.4580g. Add deionized water dropwise to the beaker containing the carbon nanotubes until the water just completely covers the sample, and let it stand for 4 hours. Use filter paper to carefully absorb the water on the surface of the sample until there are no obvious water droplets on the s...
Embodiment 2
[0041] (1) Weigh 2g of carbon nanotubes into a round bottom flask, add 100ml of concentrated nitric acid, heat and reflux in an oil bath at 140°C for 4h, then filter and wash with water until the filtrate is neutral, dry at 120°C for 12h to obtain activated of carbon nanotubes.
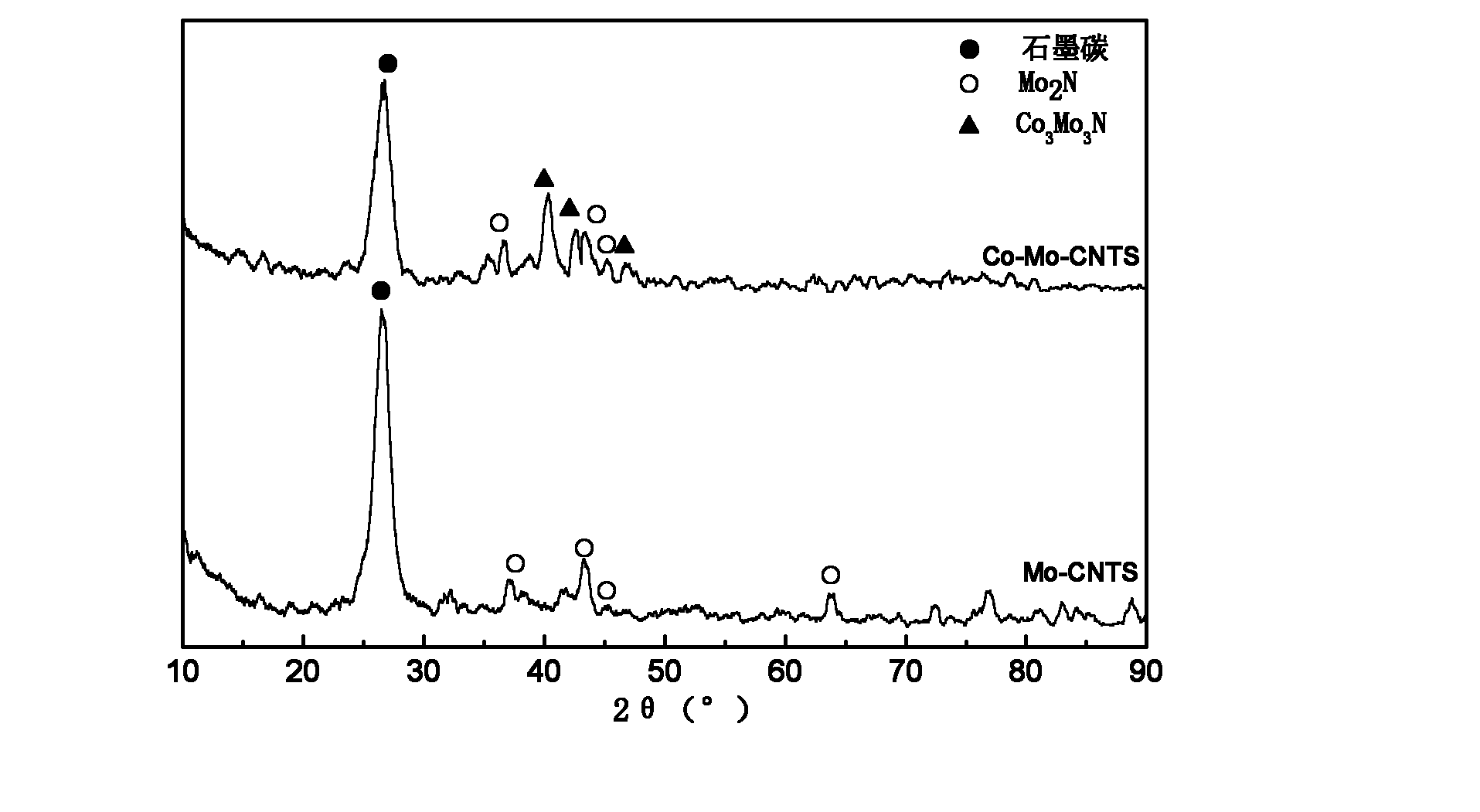
[0042] (2) The saturated water absorption of the activated carbon nanotubes is measured to be 51.0ml / g. Weigh 0.0407gCo(NO 3 ) 2 ·6H 2 O and 0.1378g (NH 4 ) 6 Mo 7 o 24 4H 2 O, dissolved in 51.0ml of distilled water to prepare the dipping solution. Mix this solution with 1.0 g of activated carbon nanotubes evenly, then let stand in the air for 12 hours, dry at 110°C for 12 hours in an air atmosphere, then calcinate at 450°C for 5 hours in a nitrogen atmosphere, and cool to room temperature to obtain the catalyst precursor, where Co 3 o 4 and MoO 3 The mass contents in the catalyst precursor are respectively 1% and 10%.
[0043] (3) Get 0.1g of the calcined product and put it into a quartz...
Embodiment 3
[0046] (1) Weigh 2g of carbon nanotubes into a round bottom flask, add 90ml of concentrated nitric acid, heat and reflux in an oil bath at 120°C for 4h, then filter and wash with water until the filtrate is neutral, and dry at 100°C for 12h to obtain activated of carbon nanotubes.
[0047] (2) The saturated water absorption of the activated carbon nanotubes is measured to be 52.5ml / g. Weigh 0.0824gCo(NO 3 ) 2 ·6H 2 O and 0.1394g (NH 4 ) 6 Mo 7 o 24 4H 2O, dissolved in 52.5ml of distilled water to prepare the dipping solution. Mix this solution with 1.0 g of activated carbon nanotubes evenly, then stand in the air for 12 hours, dry at 110°C for 12 hours in an air atmosphere, then calcinate at 500°C for 3 hours in a nitrogen atmosphere, and cool to room temperature to obtain the catalyst precursor, where Co 3 o 4 and MoO 3 The mass contents in the catalyst precursor are respectively 2% and 10%.
[0048] (3) Get 0.1g of the calcined product and put it into a quartz r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com