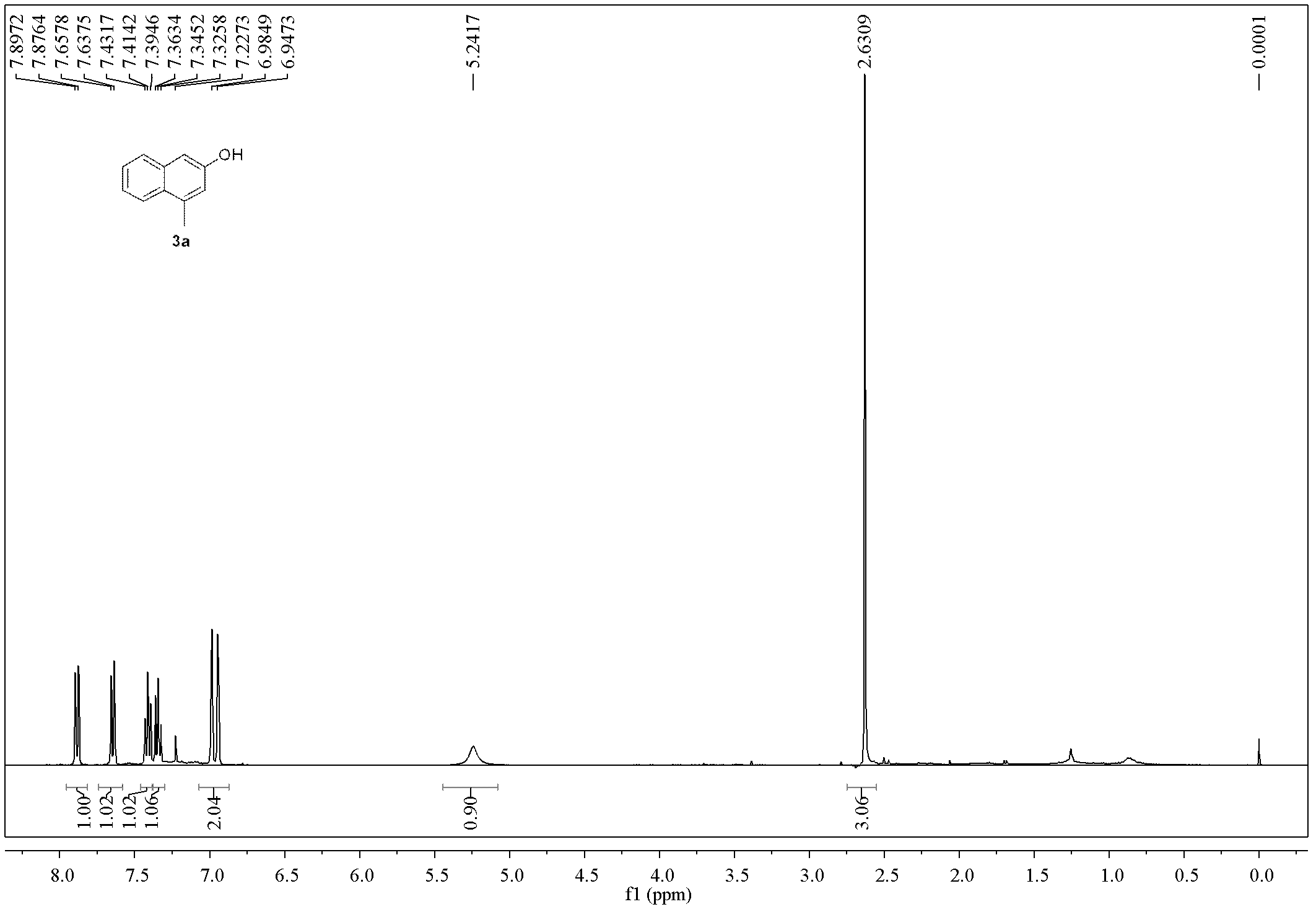
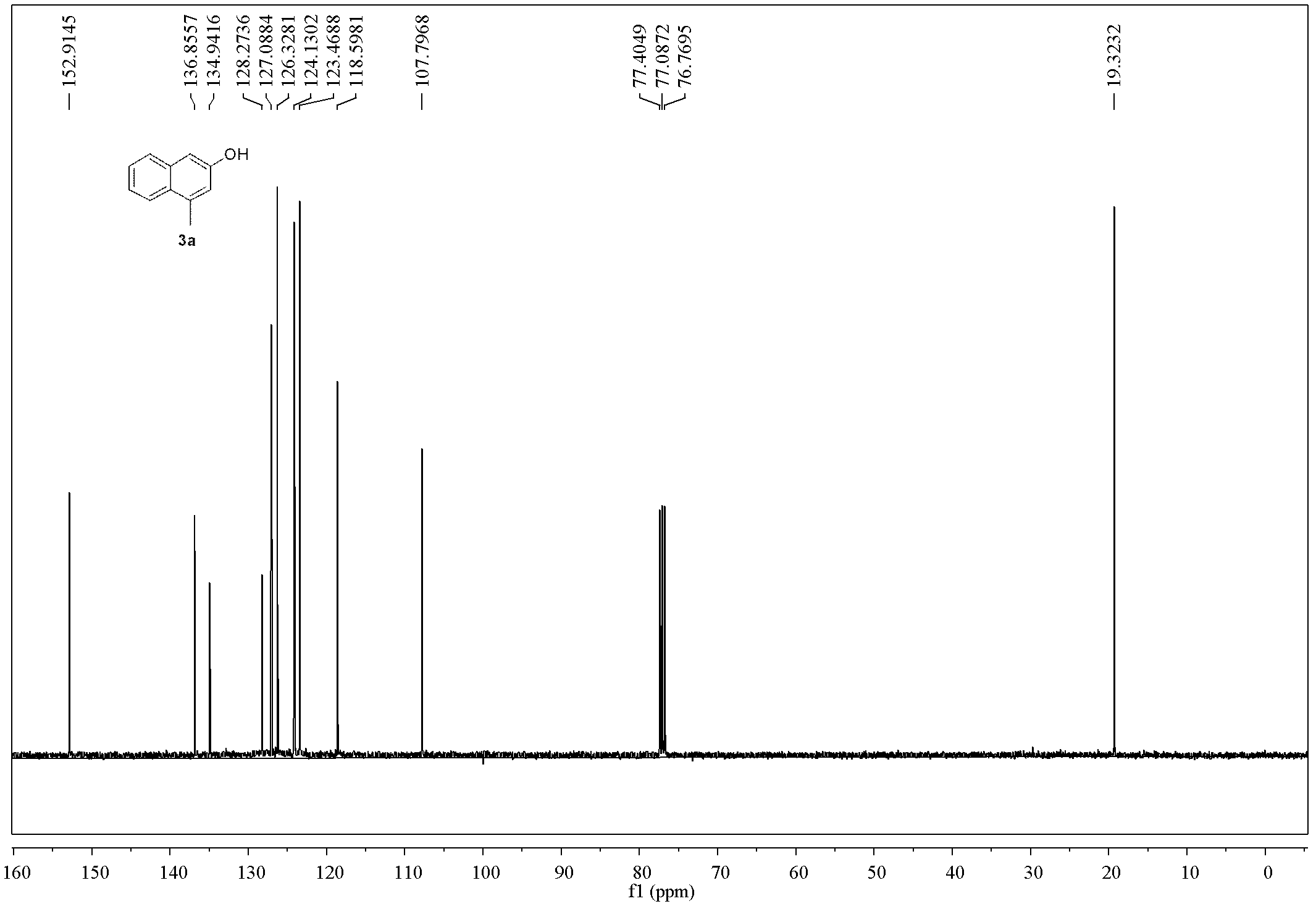
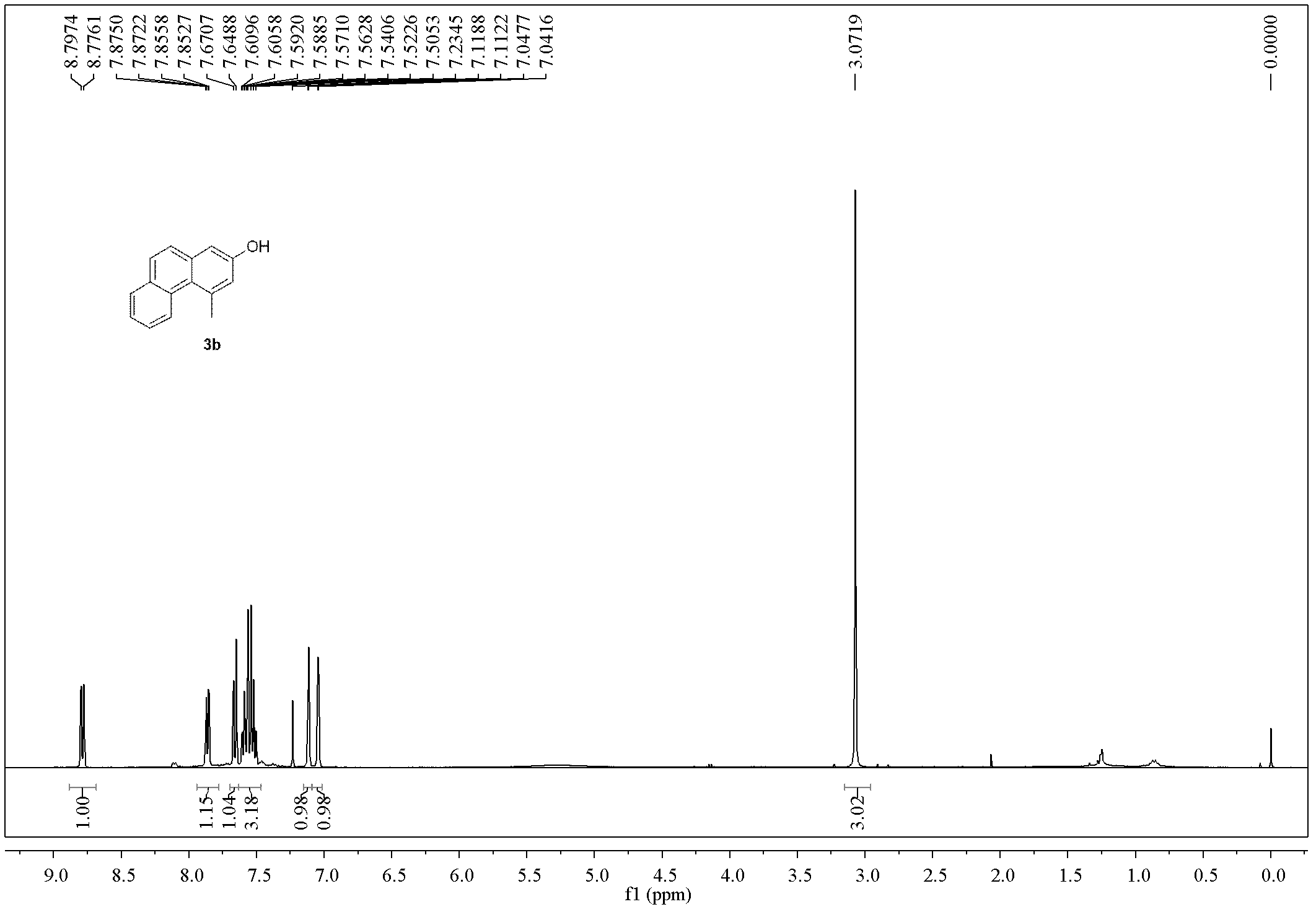
Method for preparing polysubstituted 2-naphthol
A multi-substitution, naphthol technology, applied in pharmaceutical and chemical intermediates and related chemical fields, can solve the problems of difficult synthesis of raw materials and many reaction steps, and achieve the effect of environmental friendliness and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] Add Pd(Ph 3 P) 4 (555mg, 0.48mmol, 2mol%), o-bromobenzyl bromide (1a, 5.7g, 24mmol), 1,4-dioxane (96mL), tributylallyltin (7.9g, 24mmol), and Then connect to a CO constant pressure device, put it into an 80°C oil bath and heat and stir for 12h (during which the CO pressure is kept constant at 0.2MPa). Cool to room temperature, release excess gas, remove the solvent under reduced pressure, perform direct silica gel column chromatography, elute sequentially with petroleum ether, petroleum ether: ethyl acetate = 20:1, spin dry, and dissolve the obtained product in 20 mL Ethyl acetate was passed through a layer of basic alumina to obtain 2a (4.4g of light yellow liquid, yield 76%, Z / E=20 / 80, GC ratio), and further separation by silica gel column chromatography can obtain Z and E formula respectively configuration product. 2a-Z formula, pale yellow liquid: 1 H NMR (400MHz, CDCl 3 )δ7.57(dd, J=8.0, 0.8Hz, 1H), 7.30-7.21(m, 2H), 7.15-7.11(m, 1H), 6.30-6.21(m, ...
Embodiment 2
[0058]
[0059] Add Pd(Ph 3 P) 4 (555mg, 0.48mmol, 2mol%), 1-bromo-2-naphthylbenzyl bromide (1b, 7.6g, 24mmol), toluene (96mL), tributylallyltin (7.9g, 24mmol), and then connect To the CO constant pressure device, put it into an 80°C oil bath to heat and stir for 12h (during which the CO pressure was kept constant at 0.4MPa). Cool to room temperature, release excess gas, remove the solvent under reduced pressure, perform direct silica gel column chromatography, elute sequentially with petroleum ether, petroleum ether: ethyl acetate = 20:1, spin dry, and dissolve the obtained product in 20 mL Ethyl acetate was passed through a layer of basic alumina to obtain 2a (4.4g of light yellow liquid, yield 63%, Z / E=25 / 75, GC ratio), and further separation by silica gel column chromatography can obtain Z and E respectively configuration product. 2b-Z, pale yellow solid: 1 H NMR (400MHz, CDCl 3 )δ8.31(d, J=8.5Hz, 1H), 7.83-7.77(m, 2H), 7.61-7.49(m, 2H), 7.33(d, J=8.4Hz, 1H), 6.29-...
Embodiment 3
[0062]
[0063] Add Pd(Ph 3 P) 4 (555mg, 0.48mmol, 2mol%), 2-bromo-4methylbenzyl bromide (1c, 6.7g, 24mmol), MeCN (96mL), tributylallyltin (7.9g, 24mmol), and then ligated To the CO constant pressure device, put it into an 80°C oil bath to heat and stir for 24h (during which the CO pressure was kept constant at 0.3MPa). Cool to room temperature, release excess gas, remove the solvent under reduced pressure, perform direct silica gel column chromatography, elute sequentially with petroleum ether, petroleum ether: ethyl acetate = 20:1, spin dry, and dissolve the obtained product in 20 mL Ethyl acetate was passed through a layer of basic alumina to obtain 2c (4.6g of light yellow liquid, yield 76%, Z / E=21 / 79, GC ratio), and further separation by silica gel column chromatography can obtain Z and E formula respectively configuration product. 2c-Z, pale yellow liquid: 1 H NMR (400MHz, CDCl 3 )δ7.41(s, 1H), 7.17-7.01(m, 2H), 6.35-6.15(m, 2H), 3.86(s, 2H), 2.32(s, 3H), 2.12(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com