Preparation method and application of nasopharyngeal carcinoma-relevant predisposing gene YH1 protein
A related protein, nasopharyngeal cancer technology, applied in microorganism-based methods, biochemical equipment and methods, peptide/protein components, etc., can solve problems such as no systematic report on bactericidal effect, no systematic research on the relationship between YH1 proteins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 : target gene YH1 Acquisition, identification of positive clones and construction of expression vectors
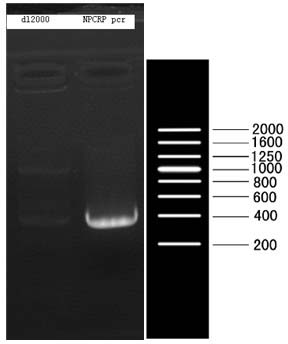
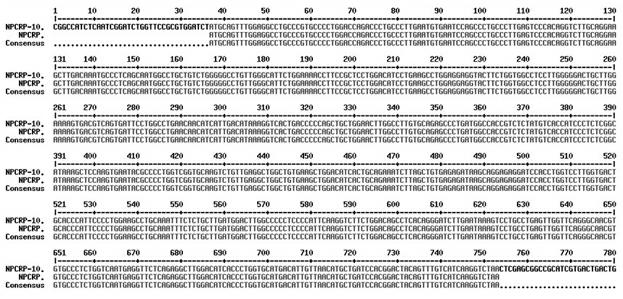
[0037] The sequence of the YH1 protein is shown below, where the 1-19 amino acids in bold italics are the signal peptide, and according to our experience in expression and purification, the signal peptide was removed when constructing the expression vector. Total RNA was extracted from nasopharyngeal tissue of patients with chronic nasopharyngitis, cDNA was reverse-transcribed with random primers, and cDNA with BglII / Xho1 double restriction sites was used YH1 PCR amplification with specific primers YH1 Gene( figure 1 ), the primer sequence is as follows (italics are restriction sites, bolded are protected bases): NPCRP-BGL2-F: 5’- CCG AGATCT ATGCAGTTTGGAGGCCTGCCCGTG-3' (SEQ ID NO.1); NPCRP-XHO-R: 5'- CCG CTCGAG TTAGACCTTGATGACAAACTGTAG-3' (SEQ ID NO. 2). The recovered product was digested with BglII / Xho1. At the same time, the pGEX...
Embodiment 2
[0043] Example 2 : Construction of expression engineered bacteria and induced expression
[0044] Extract the correct plasmid for sequencing and dissolve it in an appropriate amount of TE solution for later use. The plasmid was transformed into BL21(DE3) expression strain. Specific method: Take out the E.coli BL21 ( DE3 ) competent cells frozen at -80°C, place them on ice immediately, and transform after thawing; take 1ul of the plasmid and add it to 100uL of competent cells, mix gently, and place in the Incubate on ice for 30 min, heat shock at 42°C for 90 seconds, immediately place on ice for 3 min; add 500ul LB liquid anti-antibiotic medium preheated at 37°C, shake at 37°C, 200 rpm for 50min; take 100-200μl of bacterial solution and spread on On the LB plate containing Amp (50 μg / ml), put it into a 37°C incubator and cultivate overnight.
[0045] Take a single colony and inoculate it in 2ml LB liquid medium (containing 50 μg / ml Amp), shake and culture at 200r / min at ...
Embodiment 3
[0047] Embodiment 3: the purification of fusion protein GST-YH1
[0048] The culture supernatant was collected by centrifugation at 5000g at 4°C, then resuspended in sonication buffer (PBS, 1%Triton X-100, 1mM EDTA, pH7.4), and the lysate was sonicated 200 times at a power of 400W on ice ( Sonicate for 3 seconds at intervals of 5 seconds), then centrifuge at 15,000g for 30 minutes and take the supernatant.
[0049]Affinity chromatography: Glutathione FF chromatography column was equilibrated with buffer (PBS, 1%Triton X-100, 1mM EDTA, pH7.4) for 10 column volumes, and then the bacterial supernatant was loaded onto the chromatography column, and then buffer ( PBS, 1mM EDTA, pH7.4) was washed for 20 column volumes, and then GST-YH1 was eluted with buffer (PBS, 10mM GSH, 1mM EDTA, pH8.0). The induced supernatant, precipitate and eluate were taken respectively for SDS-PAGE electrophoresis analysis ( Figure 6 ). The purified GST-YH1 fusion protein was stored at -80 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com