Method for preparing boron trifluoride used as catalyst
A boron trifluoride and catalyst technology, applied in the direction of boron halide compounds, etc., can solve the problems of cumbersome process and proportion error affecting the bonding quality, etc., achieve good use of cold and heat, solve the problem of unstable production, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
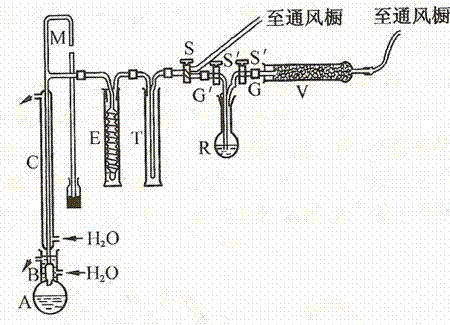
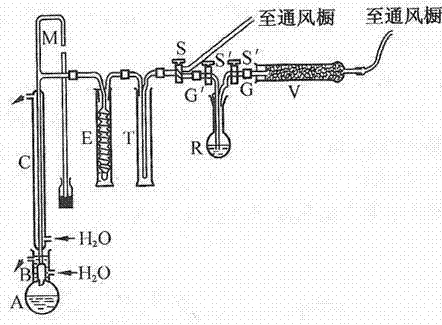
[0052] Grind 300 grams of sodium fluoroboride and 50 grams of anhydrous boric anhydride together, and then put the mixture in a flask; add 300 milliliters of concentrated sulfuric acid, shake the flask to fully mix the solid and liquid, and reduce the temperature at 130 ° C. It is produced by removing excess boron trifluoride under pressure. See the process for details figure 1 .
Embodiment 2
[0054] Grind 350 grams of sodium fluoroboride and 60 grams of anhydrous boric anhydride together, and then put the mixture in a flask; add 500 milliliters of concentrated sulfuric acid, shake the flask to fully mix the solid and liquid, and reduce the temperature at 130 ° C. It is produced by removing excess boron trifluoride under pressure. See the process for details figure 1 .
Embodiment 3
[0056] Sodium fluoroborate (350 g addition) and anhydrous boric anhydride (60 g addition) were ground together and the mixture was placed in a flask. Add about 300 ml of concentrated sulfuric acid, shake the flask to fully mix the solid and liquid, use a grease on the standard conical interface B (saturated with boron trifluoride 1:4 paraffin and vaseline mixture, And it is made under reduced pressure at 130°C to remove excess boron trifluoride) Connect the flask A to the device, open the piston D, and evacuate the system to vacuum; then close the piston D to make the boron trifluoride produced Through washing bottle E, the concentrated sulfuric acid saturated with boric anhydride is housed in the washing bottle E, to wash away hydrofluoric acid and moisture. The flask was heated slightly. Foaming occurred and when the foaming ceased, the reaction mixture was heated vigorously. Boron trifluoride is condensed in several ampoules F, and the outside of one ampoule is soaked w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com