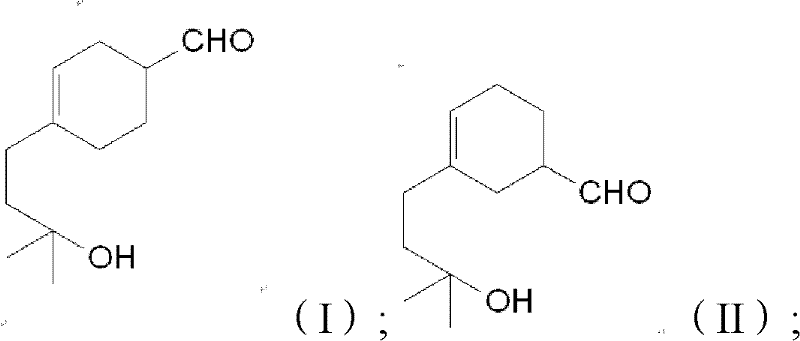
Preparation method of lyral
A technology of lyral and acrolein, which is applied in the field of preparation of lyral, can solve the problems of waste acid corrosion, environmental pollution, cumbersome operation steps, etc., and achieve few by-products, high yield and purity, and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of lyral, comprising the following steps:
[0027] a) Myrcenol is heated to 50°C to 130°C;
[0028] b) adding acrolein dropwise to the myrcenol obtained in step a), controlling the temperature during the dropping process to be 50° C. to 130° C., and continuing the reaction after the dropwise addition to obtain neoliral.
[0029] In the present invention, firstly, the temperature of myrcenol is raised to 50°C to 130°C, that is, to the reaction temperature of myrcenol and acrolein, so that the subsequent dropwise added acrolein can immediately react with myrcenol, and the temperature is preferably 70°C ~120°C, more preferably 100°C to 110°C.
[0030] Myrcenol is a diene body with a conjugated double bond, which is prone to self-polymerization. In order to prevent myrcenyl from self-polymerization, the myrcenol preferably contains a polymerization inhibitor, and the polymerization inhibitor is preferably p-phenylene. Di...
Embodiment 1
[0045] Add 539g (3.5mol) myrcenol and 0.539g hydroquinone in the 1000mL four-necked flask that thermometer, mechanical stirring device and reflux condenser are equipped with, be heated to 110 ℃; In described myrcenol, dropwise add 235g ( 4.2 mol) of acrolein was added dropwise for 6 hours. During the dropwise addition, the reaction bottle was placed in a freezing tank, and the temperature of the reaction bottle was kept at 110°C to 130°C; after the addition of acrolein was completed, the reaction was continued for 1h to obtain the reaction mixture;
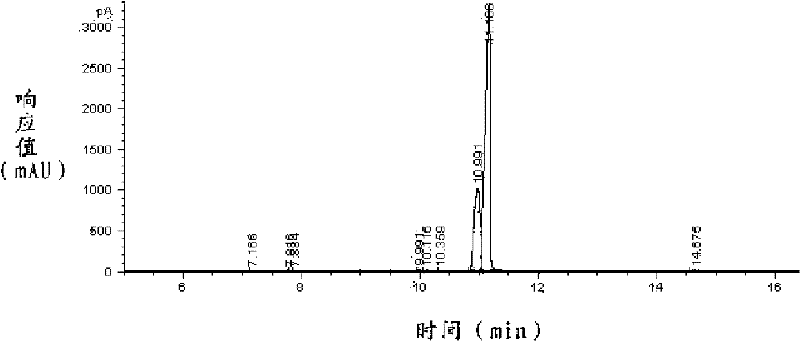
[0046] The reaction mixture is washed three times with 200g deionized water to obtain the crude product of 770g lyral, and the lyral is carried out to gas chromatography analysis, wherein the content of lyral is 96.6%; Temperature below 2mmHg carries out rectification, collects the cut between 120 ℃~125 ℃, obtains 616g lyral.
[0047] Described lyral is carried out gas chromatography analysis, the result sees figure 1 with fig...
Embodiment 2
[0051] Add 539g (3.5mol) myrcenyl alcohol and 0.539g hydroquinone in the 1000mL four-necked flask that thermometer, mechanical stirring device and reflux condenser are equipped with, be heated to 110 ℃; In described myrcenyl alcohol, dropwise add 216g ( 3.86 mol) of acrolein, added dropwise for 6 hours. During the dropwise addition, the reaction bottle was placed in a freezing tank, and the temperature of the reaction bottle was kept at 110°C to 130°C; after the dropwise addition of acrolein was completed, the reaction was continued for 1 hour to obtain mixture;
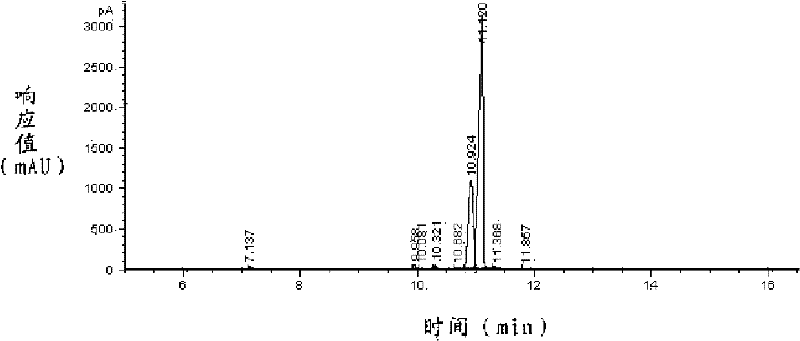
[0052] The reaction mixture is washed three times with 180g deionized water to obtain the crude product of 761g lyral, and the lyral is carried out to gas chromatography analysis, wherein the content of lyral is 95.2%; Temperature below 2mmHg, carry out rectification, collect the distillate between 120 ℃~125 ℃, obtain 605g lyral.
[0053] The lyral was analyzed by gas chromatography, and its main peaks appeared at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com