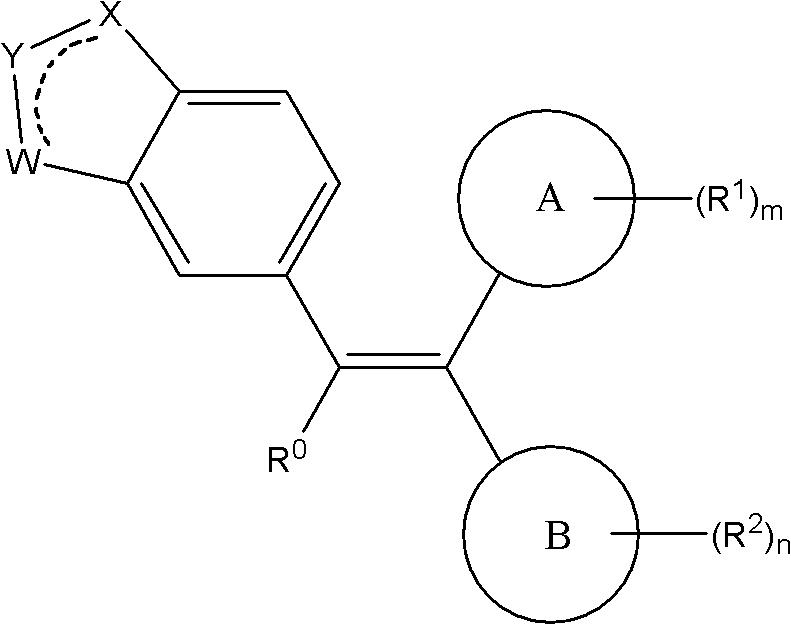
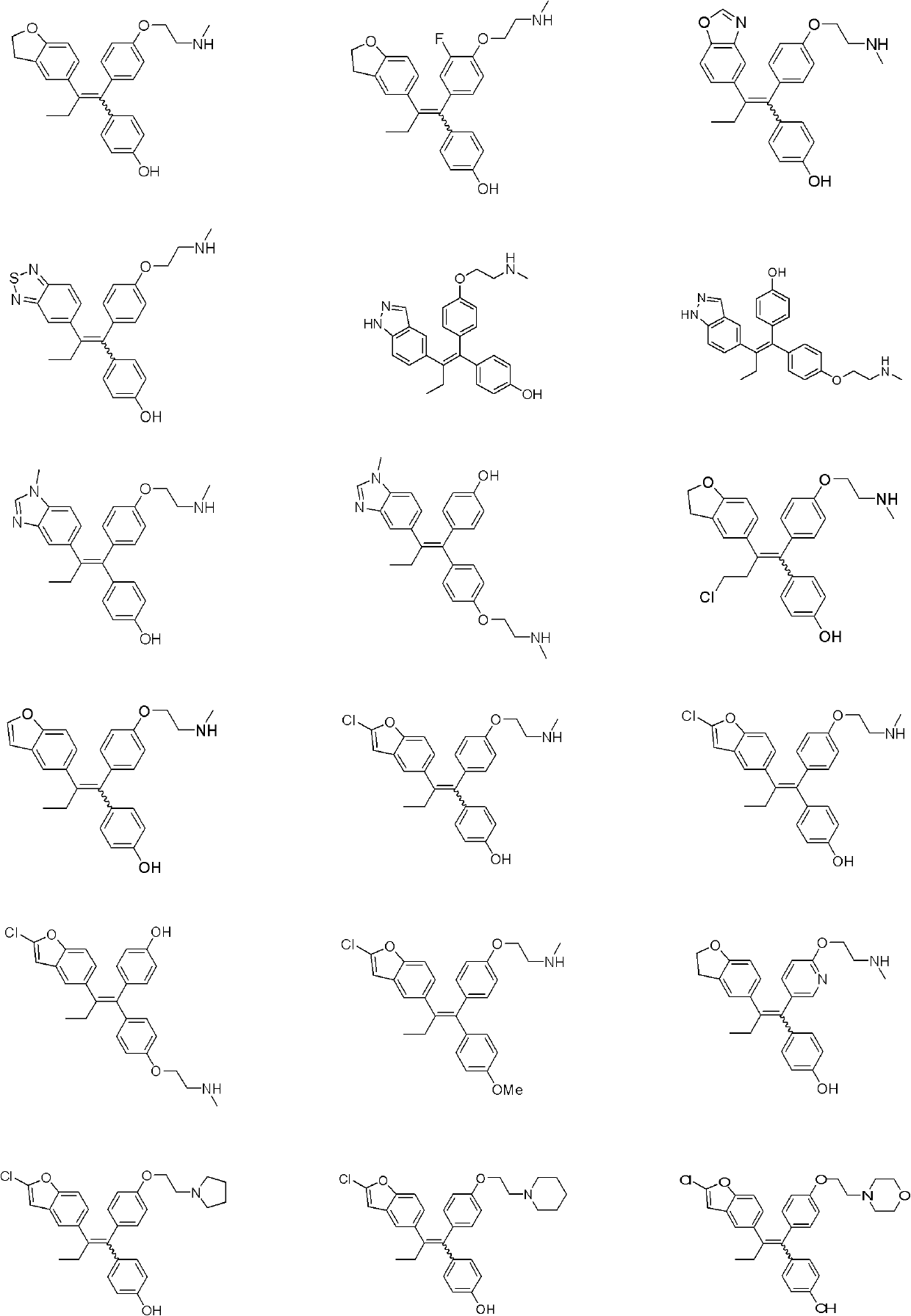
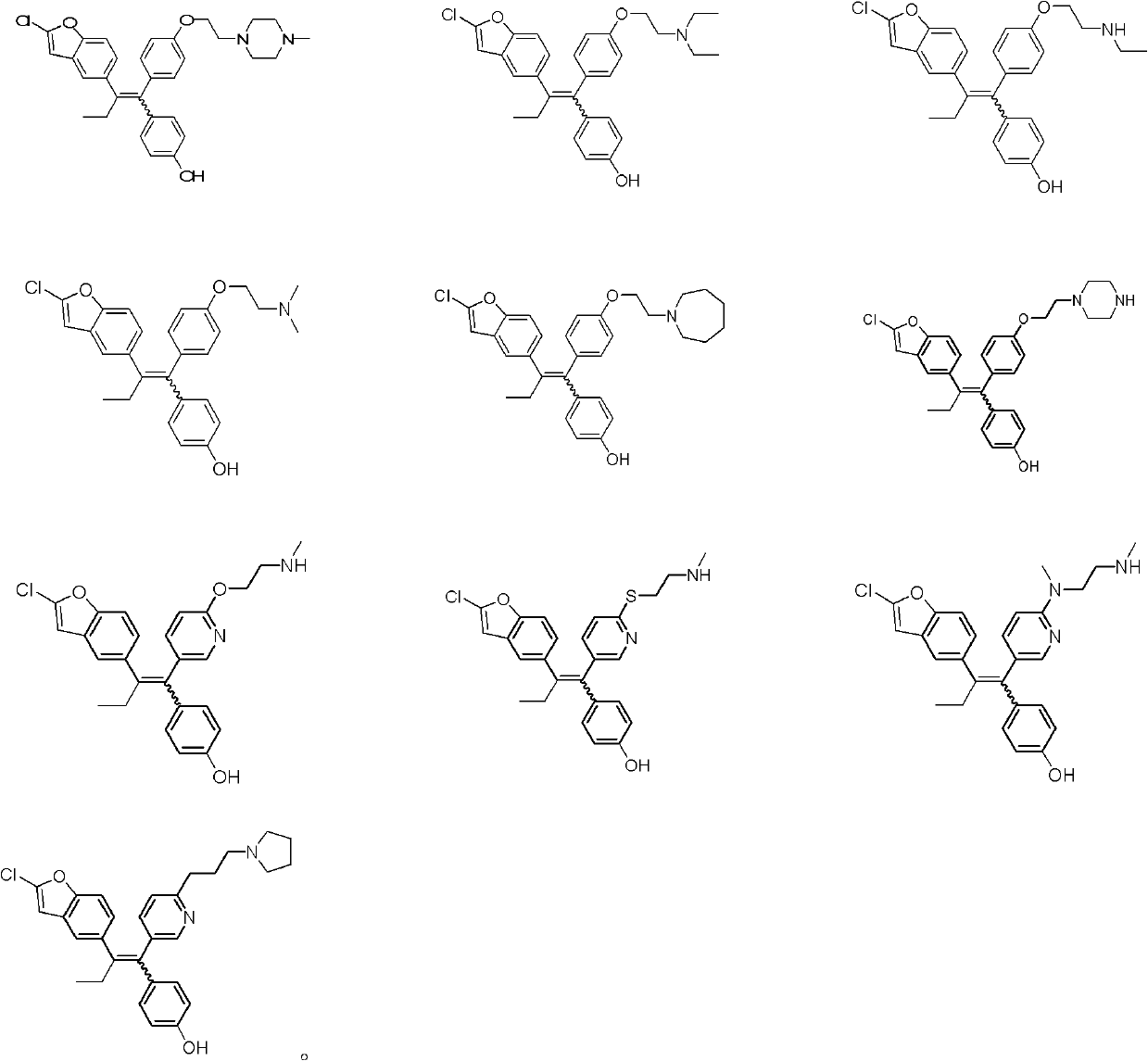
Ethylene derivative serving as selective estrogen receptor modulators (SERMs)
A solvate and selected technology, applied in the field of ethylene derivatives, can solve the problem of poor clinical effect of breast cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] Preparation of compounds of the present invention
[0114] The following reaction schemes illustrate the preparation of the compounds of the invention.
[0115] It should be understood by those skilled in the art that in the following description, combinations of substituents are permissible only when such combination can result in a stable compound.
[0116] Those skilled in the art will also understand that in the methods described below, the functional groups of intermediate compounds may need to be protected by appropriate protecting groups. Such functional groups include hydroxyl, amino, mercapto and carboxylic acid. Suitable hydroxy protecting groups include trialkylsilyl or diarylalkylsilyl groups (eg tert-butyldimethylsilyl, tert-butyldiphenylsilyl or trimethylsilyl) , tetrahydropyranyl, benzyl, etc. Suitable protecting groups for amino, amidino and guanidino include tert-butoxycarbonyl, benzyloxycarbonyl and the like. Suitable protecting groups for mercapt...
Embodiment 1
[0126] 4-[2-(2,3-Dihydro-5-benzofuryl)-1-{4-[2-(methylamino)ethoxy]phenyl}but-1-ene]phenol
[0127]
[0128] Step A: 1-(2,3-Dihydrobenzofuran-5-yl)propyl-1-one
[0129]
[0130] Dissolve 2,3-dihydrobenzofuran (10 g, 1.0 equivalent) in 200 mL of dichloromethane, and add propionyl chloride (14 g, 1.8 equivalent) and anhydrous aluminum chloride (11.1 g , 1.0 equivalent) in 200 mL of dichloromethane solution. Stir at room temperature for 1 hour after the dropwise addition, add ice water to quench the reaction, extract the aqueous phase with dichloromethane, combine the organic phases and dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a crude product. The crude product was added with n-hexane (100 mL), cooled to 0°C, stirred for 30 minutes, the suspension was filtered with suction and washed with cold n-hexane to obtain the title compound (11.8 g, 80%).
[0131] 1 H NMR (400MHz, CDCl 3 )δ7.86(s, 1H), 7.81(d, J=8.4Hz, 1H), 6.80(...
Embodiment 2
[0148] (Z)-4-(2-(1H-indazol-5-yl)-1-{4-[2-(methylamino)ethoxy]phenyl}but-1-enyl)phenol
[0149]
[0150] Step A: Methyl 4-amino-3-methylbenzoate
[0151]
[0152]At room temperature, add methyl 4-nitro-3-methylbenzoate (50g, 1.0 equivalent) into 1L of methanol solution, install a mechanical stirrer, add ammonium chloride (137g, 10 equivalent) and dissolve the solid in 60mL of water of aqueous solution. Iron powder (96 g, 7 equivalents) was added, and the reaction mixture was refluxed at 75° C. for 4 hours. After the reaction, it was cooled to room temperature, filtered, washed with water, extracted with ethyl acetate, dried and concentrated to directly obtain the title compound (26 g, 62%) as a white solid.
[0153] Step B: Methyl 4-diazotetrafluoroborate-3-methylbenzoate
[0154]
[0155] At room temperature, add 4-amino-3-methylbenzoic acid methyl ester (15g, 1.0 equivalent) into tetrafluoroboric acid solution (40% aqueous solution), cool to 0°C with an ice bath,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com