Oligopeptide with breast cancer resistant activity and application thereof
An anti-breast cancer and breast cancer cell technology, applied in oligopeptides and their applications, oligopeptides with anti-breast cancer activity and their applications, can solve the problems of long cycle and high cost, and achieve growth inhibition, small molecular weight, and inhibition proliferating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Design and synthesis of oligopeptides
[0030] 1. Extract the major international antimicrobial peptide database (http: / / aps.unmc.edu / AP / main.php; http: / / penbase.immunaqua.com / ; http: / / www.bbcm.univ.trieste.it / ; Http: / / www.imtech.res.in / raghava / antibp / ), analyze the corresponding relationship between its primary structure and activity, and design the artificial short peptide sequence.
[0031] 2. Use the protein analysis database http: / / www.expasy.org / to analyze the similarity between the oligopeptide sequence of known activity and the secondary structure of the designed oligopeptide sequence, and combine other physical and chemical parameter predictions to modify the design sequence .
[0032] 3. Send the oligopeptide sequence to the biological company for synthesis or use an automatic oligopeptide synthesizer to synthesize the full sequence. HPLC detection purity is greater than 96%, and mass spectrometry is used to determine the quality of oligopeptide synthesis. The s...
Embodiment 2
[0037] Identification of oligopeptides
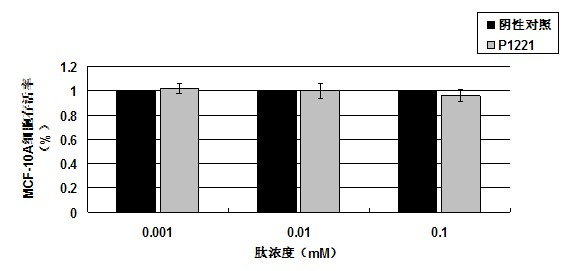
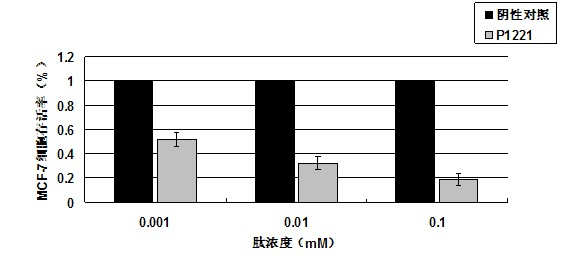
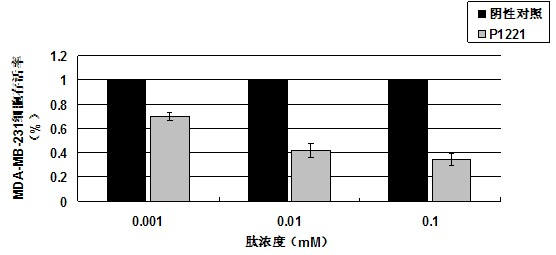
[0038] P1221 oligopeptide was hydrolyzed by 5.7mol / L HCl for 16 hours and analyzed for amino acid. It was found that it contained 9 kinds of amino acids, including His, Asn, Tyr, Gly, Phe, Val, Pro, Ser, Leu, and its molar ratio was His:Asn : Tyr: Gly: Phe: Val: Pro: Ser: Leu = 1: 1: 1: 1: 1: 1: 1: 1: 1 After amino acid sequence analysis, the sequence is His-Asn-Tyr-Gly-Phe-Val-Pro-Ser-Leu. A peak was determined by electrospray mass spectrometry, and its molecular weight was 1033.15.
[0039]
Embodiment 3
[0041] Detection of tumor suppressor activity of P1221 oligopeptide in vitro by MTT method
[0042] 2.1 Materials and reagents
[0043] 1) P1221 oligopeptide, synthesized by Shanghai Shenggong Biological Engineering Technology Service Co., Ltd.
[0044] 2) Human breast cancer cell lines MCF-7, MDA-MB-231, and human normal breast cells MCF-10A were purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0045] 3) RPMI 1640 medium, DMEM medium, and newborn calf serum are all products of GIBCO, USA.
[0046] 4) Dimethyl sulfoxide (DMSO), tetramethyl azoazole (MTT) and trypsin are products of Sigma.
[0047]
[0048] 2.2 Experiment
[0049] 1) Dilute P1221 oligopeptide to an initial concentration of 1mM with diluent (sterile PBS, or sterile water, or corresponding cell culture medium), filter and sterilize, and freeze at -20°C after packaging.
[0050] 2) Cell culture: The cancer cells are cultured in RPMI 1640 culture medium (contain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com