Preparation and application of brucella gene engineering subunit vaccines
A Brucella, subunit technology, applied in bacteria, fungi, antibacterial drugs, etc., can solve problems such as no vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 The source of the fusion protein gene
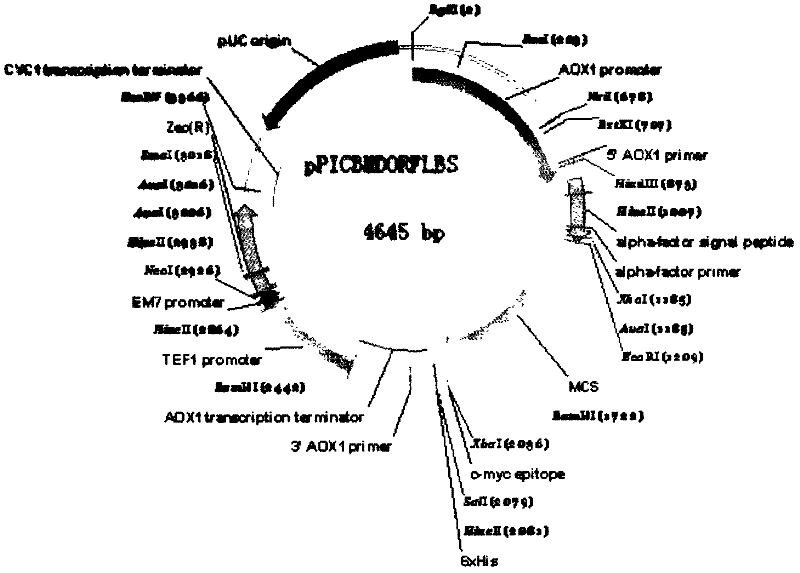
[0049] The coding gene of the entire fusion protein is artificially designed according to the preferred codons of Escherichia coli, and is named as the ORF-BLS fragment (it has a sequence of 1-969 nucleotide sequences in SEQ ID No.1 and submitted to Shanghai Handsome Biotechnology Co., Ltd. Synthesized by the company, the nucleotide sequence at position 970-1035 in SEQ ID No.1 is provided by the nucleotide sequence at position 1277-1340 of the vector pPICZalpha A of Invitrogen Company. EcoRI ( 5' end) and XbaI (3' end) restriction endonuclease sites. Shanghai Handsome Biotechnology Co., Ltd. synthesized this fragment and cloned it into the pMD18T vector, transformed Escherichia coli, extracted and sequenced correctly, and named this fragment as The Escherichia coli of the pMD18T-ORF-PBLS recombinant plasmid was sent to our company.
Embodiment 2
[0050] Example 2 Construction of Fusion Protein Yeast Expression Vector
[0051] The Escherichia coli strain containing the recombinant plasmid named pMD18T-ORF-PBLS was inoculated into the 10mlLB medium containing ampicillin 50mg / L, and the Escherichia coli strain containing the Pichia pastoris secretion expression vector pPICZalphaA (purchased from Invitrogen Company), Inoculate into low-salt LB medium containing 25mg / L Zeocin, culture overnight at 37°C with shaking, and extract plasmids respectively according to the instruction manual of Qiagen plasmid extraction kit the next day. Recombinant plasmids containing genes encoded by fusion fragments were treated with restriction endonucleases corresponding to both ends of the fragments, and pPICZalpha A, a secretion expression vector of Pichia pastoris, was treated with EcoRI and XbaI. Specific treatment conditions: 10l reaction system, 2l plasmids were added to the system , each restriction endonuclease used 5 activity units (...
Embodiment 3
[0055] Example 3 Construction and Screening of Fusion Protein Expression Strains
[0056] Inoculate the above-mentioned positive clones into 50ml low-salt LB medium containing 25mg / L Zeocin. After 8-12 hours, transfer to 500-1000ml low-salt LB medium containing 25mg / L Zeocin, cultivate overnight, and extract a large number of plasmids for later use. .
[0057] Preparation of linearized DNA: In a 100 l reaction system, add 20 g of the DNA extracted above, add Pme I 20∪ (New England biolabs), and make up with deionized water. Digest at 37°C for 3 hours. Take 2 liters of digested products and run them on 1% agarose gel electrophoresis to observe whether the digested enzymes are complete. After confirming that the linearization is complete, add 2l of 200mM EDTA to the remaining digested product to terminate the reaction.
[0058] Purification of linearized DNA: Add 100l of deionized water to the above enzyme digestion system, add an equal volume of phenol / chloroform, shake vigo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com