Preparation method for nano ferroferric oxide
A ferric tetroxide and nanotechnology, applied in the direction of iron oxide/ferric hydroxide, nanotechnology, ferrous oxide, etc., can solve the problems of wide particle size range and large particle size of ferric tetroxide, and achieve Narrow particle size range and uniform particle size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) 0.4705g (0.0012mol) FeSO 4 ·(NH 4 ) 2 SO 4 ·6H 2 O and 0.5406g (0.0020mol) FeCl 3 ·6H 2 O was dissolved in 100ml of distilled water to obtain liquid A; 2.0g of hydrazine hydrate was dissolved in 50ml of distilled water to obtain liquid B. Put liquid A in a flask, under the protection of argon, and stir at a temperature of 2°C, use a dropping funnel to add liquid B dropwise into liquid A, after 0.5 hours, continue to stir and react at a temperature of 2°C for 1 hour, After the reaction was completed, the reaction suspension was added to the centrifuge tube, and after centrifugation at 6000 rpm for 5 minutes, the upper liquid was removed to obtain a solid, and the solid was washed with distilled water until the pH of the supernatant in the centrifuge tube was 7, and the solid was separated. And dry the solid to give FeO(OH).
[0024] Structure Identification
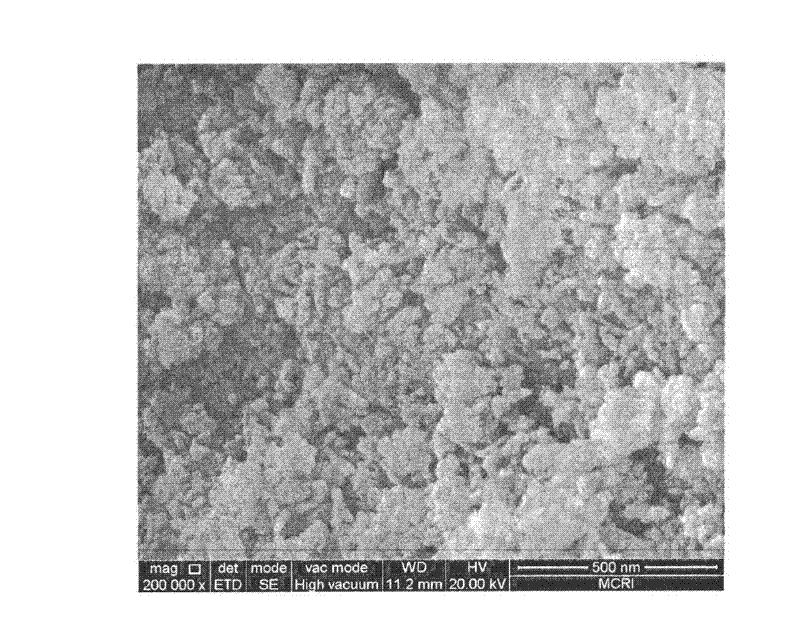
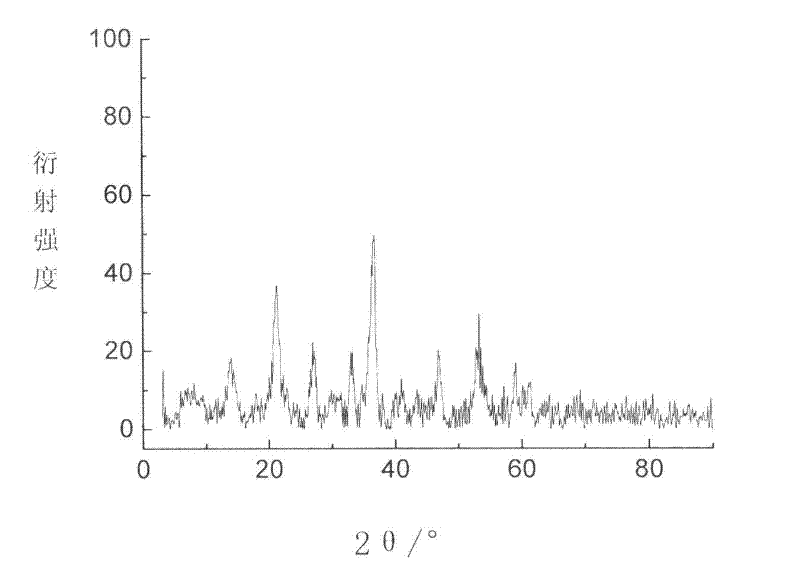
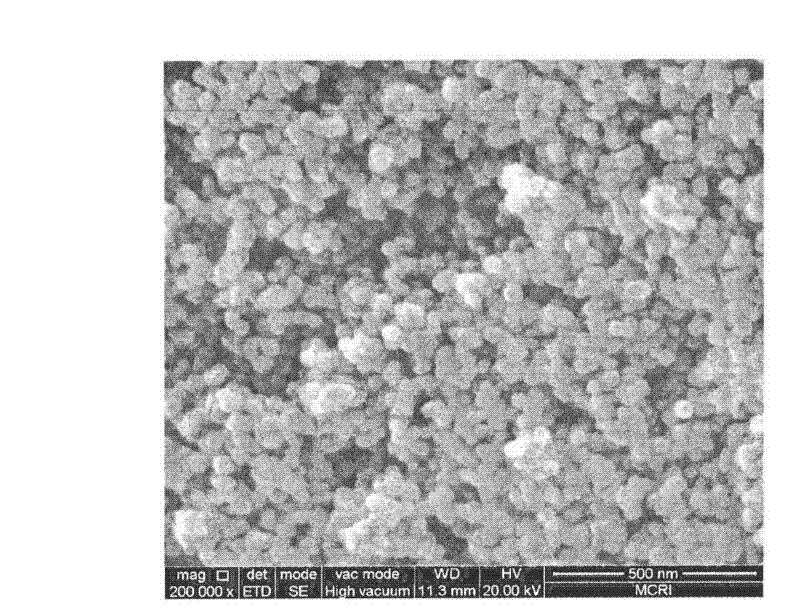
[0025] It was confirmed by XRD detection that the solid obtained by the above method is indeed FeO(OH)...
Embodiment 2
[0030] 1) Add 0.2780g (0.0010mol) FeSO 4 ·7H 2 O and 0.3244g (0.0020mol) FeCl 3 Dissolve in 100ml of distilled water to obtain liquid A; dissolve 6.7g of hydrazine hydrochloride in 50ml of distilled water to obtain liquid B. Put liquid A in a flask, under the protection of argon, and stir at a temperature of 25°C, use a dropping funnel to add liquid B dropwise into liquid A, drop it in 2 hours, continue to stir and react at a temperature of 25°C for 5 hours, After the reaction was completed, the reaction suspension was added to the centrifuge tube, and after centrifugation at 6000 rpm for 5 minutes, the upper liquid was removed to obtain a solid, and the solid was washed with distilled water until the pH of the supernatant in the centrifuge tube was 7, and the solid was separated. And dry the solid to give FeO(OH).
[0031] 2) Add 0.1g FeO(OH) obtained in step 1) into the hydrothermal kettle, add 20ml of 0.5% (weight) NaOH aqueous solution, react at 100°C for 10 hours, cool...
Embodiment 3
[0033] 1) Add 0.2780g (0.0010mol) FeSO 4 ·7H 2 O and 0.4040g (0.0010mol) Fe(NO 3 ) 3 9H 2 O was dissolved in 100ml of distilled water to obtain liquid A; 6.7g of hydrazine hydrochloride was dissolved in 50ml of distilled water to obtain liquid B. Put liquid A in a flask, under the protection of nitrogen, and stir at a temperature of 15°C, use a dropping funnel to add liquid B dropwise into liquid A, after 1.5 hours, continue to stir and react at a temperature of 15°C for 3 hours. After completion, the reaction suspension was added to the centrifuge tube, and after centrifugation at 6000 rpm for 5 minutes, the upper liquid was removed to obtain a solid, and the solid was washed with distilled water until the pH of the supernatant in the centrifuge tube was 7, and the solid was separated and The solid was dried to give FeO(OH).
[0034]2) Add 0.1g FeO(OH) obtained in step 1) into the hydrothermal kettle, add 20ml of 0.8% (weight) KOH aqueous solution, react at 150°C for 18 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com