Method for producing p-tert-Butyltoluene by using mordenite for catalyzing toluene and tertiary butanol
A technology of p-tert-butyltoluene and mordenite, which is applied in the field of production of p-tert-butyltoluene, can solve the problems of environmental pollution, high production intensity, and low yield, and achieve the effect of overcoming corrosion of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
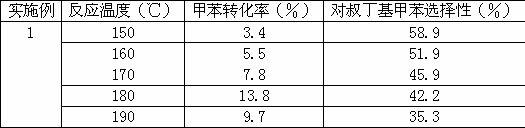
[0012] Reaction conditions: the molar ratio of raw materials is 1:4 (toluene: tert-butanol), and the volumetric liquid space velocity is 2 ml / g cat ·H, the catalyst is a mordenite molecular sieve catalyst that has been activated at 550°C for 3 hours. The gas-solid phase continuous reaction is carried out at different reaction temperatures for 10 hours. The results are shown in Table 1.
[0013] Attached Table 1 Catalytic performance at different reaction temperatures
[0014]
Embodiment 2
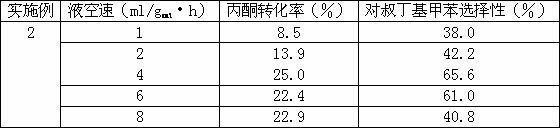
[0016] Reaction conditions: The reaction temperature is 180℃, the molar ratio of raw materials (toluene: tert-butanol) is 1:4, the catalyst is mordenite molecular sieve catalyst which has been activated at 550℃ for 3 hours, and the gas-solid phase continuous reaction is carried out at different liquid space velocities. 10 hours, the results are shown in Table 2.
[0017] Attached Table 2 Catalytic performance under different liquid space velocity
[0018]
Embodiment 3
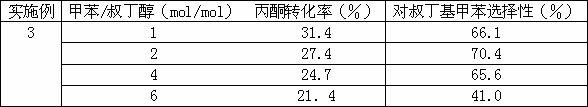
[0020] Reaction conditions: feed space velocity 4 ml / g cat ·H. The reaction temperature is 180°C. The catalyst is a mordenite molecular sieve catalyst that has been activated at 550°C for 3 hours. Different raw material ratios (toluene: tert-butanol) are used for continuous gas-solid reaction for 10 hours. The results are shown in Table 3.
[0021] Attached Table 3 Catalytic performance under different toluene / tert-butanol ratios
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com