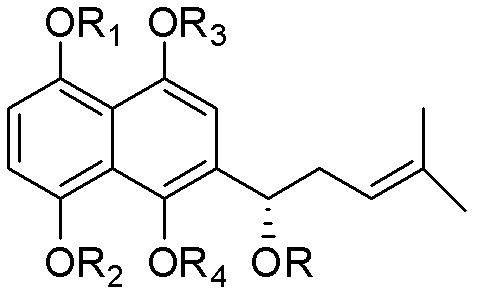
Naphthazarin nucleus oxialkyl and acyl substituted alkannin derivative, its preparation and application thereof
A kind of nucleooxyalkyl, acyl substitution technology, applied in the field of chiral natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
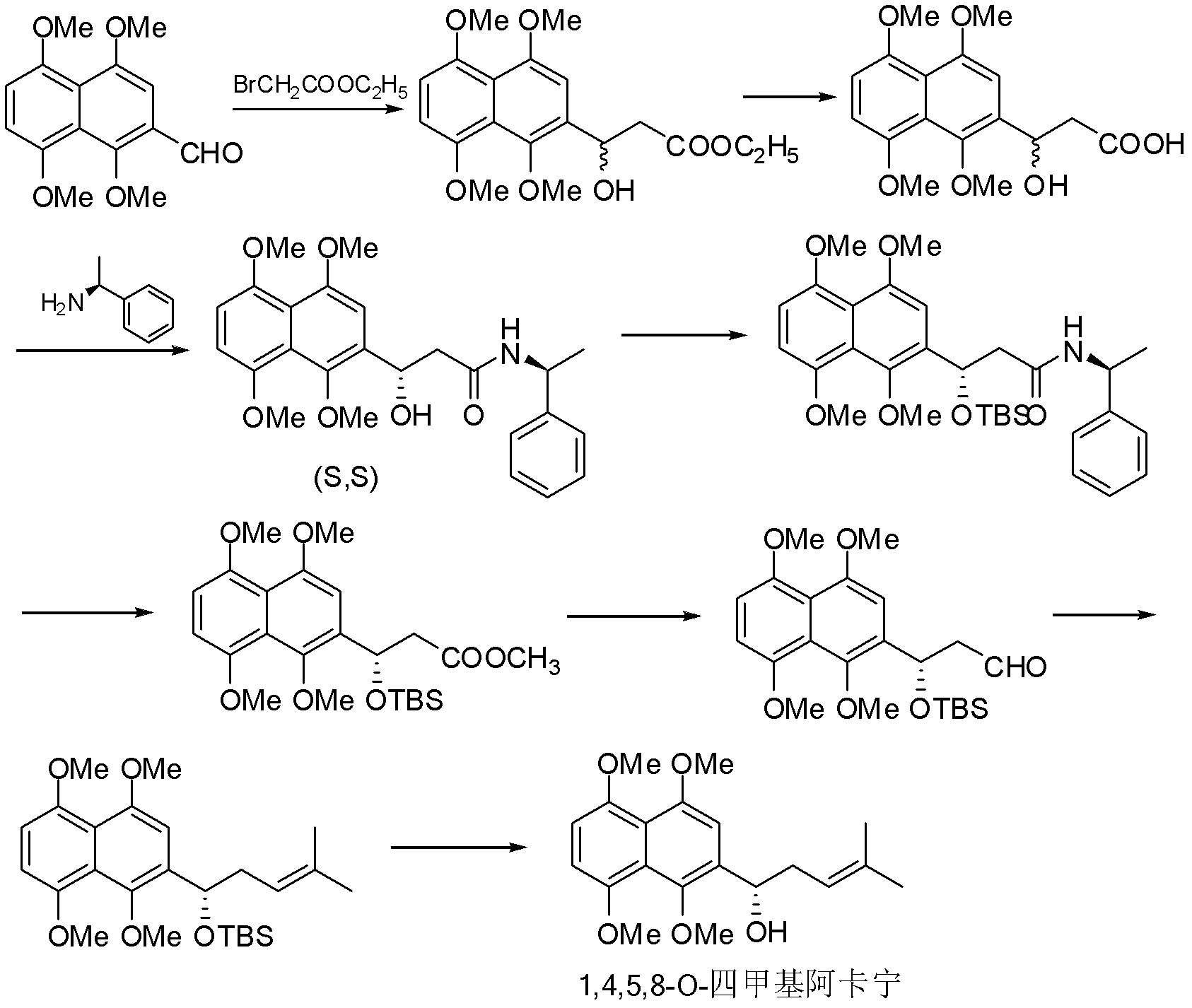
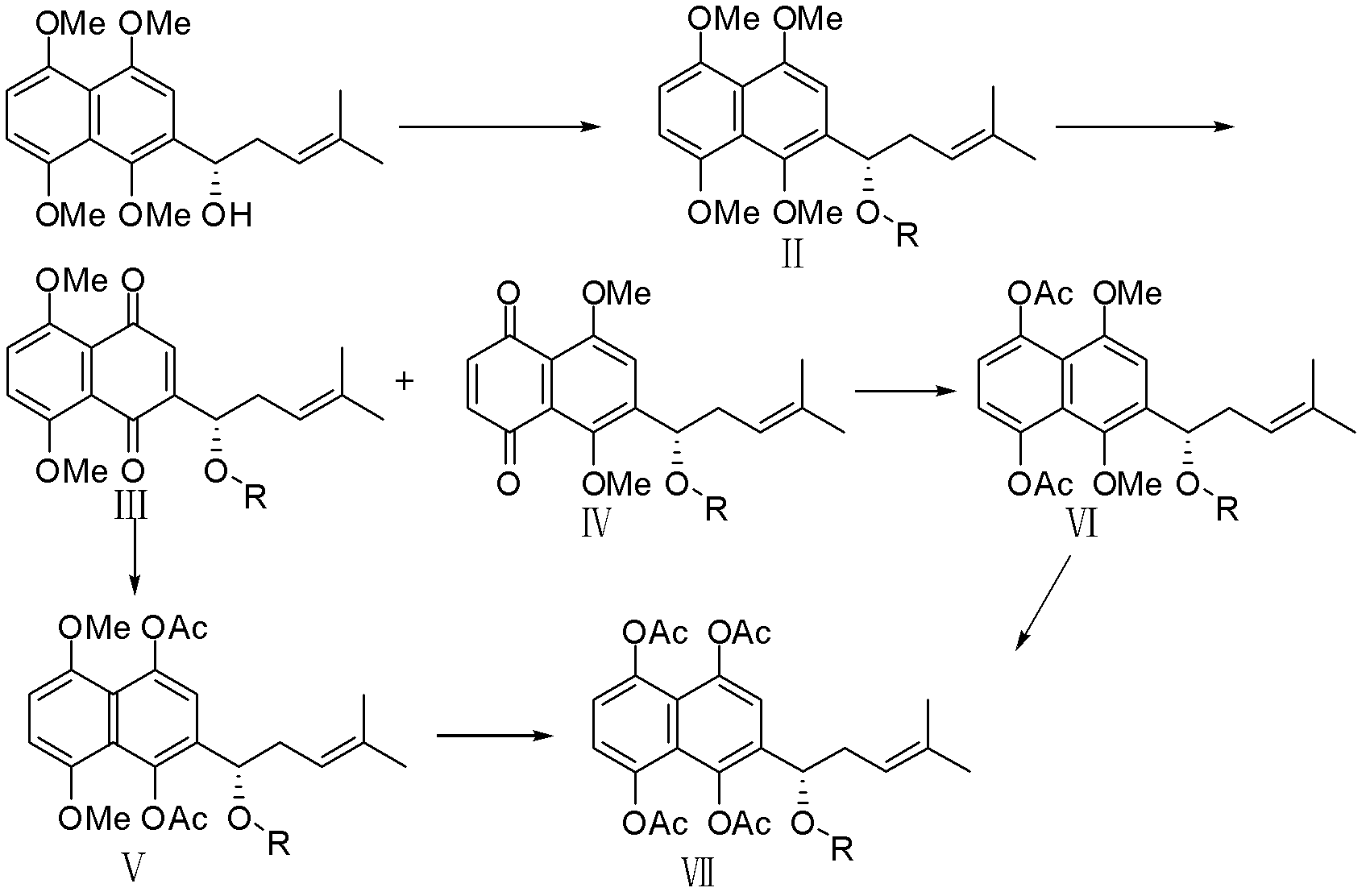
[0047] Compound (s)-2-(1-acetoxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (II-1), (s)-2 -(1-acetoxy-4-methyl-3-pentenyl)-5,8-dimethoxy-1,4-naphthoquinone (III-1) and (s)-6-(1- Preparation of Acetoxy-4-methyl-3-pentenyl)-5,8-dimethoxy-1,4-naphthoquinone (IV-1)
[0048] (s)-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (1,4,5,8-O-tetramethyl Arkanin) was dissolved in anhydrous dichloromethane, and 1.5 equivalents of acetic anhydride, 2 equivalents of triethylamine, and a catalytic amount of DMAP were added at 0-5°C, stirred at room temperature for 15-30 minutes, and extracted with dichloromethane. Dry over anhydrous sodium sulfate, concentrate and column chromatography to obtain compound II-1 as a yellow oil. Yield 96%, 1 H NMR (300MHz, CDCl 3 ): δ=6.87(s, 1H, H Ar ), 6.82(s, 2H, H Ar ), 6.34(dd, 1H, J=4.2, 6.0Hz, -CH-), 6.15(t, 1H, J=4.5Hz, -CH=), 3.93(s, 6H, 2×-OCH 3 ), 3.86(s, 3H, -OCH 3 ), 3.83 (s, 3H, -OCH 3 ), 2.54-2.95 (m, 2H, -CH ...
Embodiment 2
[0053] Compound (s)-2-[1-(3-methyl-but-2-enoyloxy)-4-methyl-pent-3-enyl]-1,4,5,8-tetramethoxy Base naphthalene (II-2), (s)-2-[1-(3-methyl-but-2-enoyloxy)-4-methyl-pent-3-enyl]-5,8- Dimethoxy-1,4-naphthoquinone (III-2) and (s)-6-[1-(3-methyl-but-2-enoyloxy)-4-methyl-pent-3 Preparation of -alkenyl]-5,8-dimethoxy-1,4-naphthoquinone (IV-2)
[0054] Dissolve (s)-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene in anhydrous dichloromethane at 0-5 ℃ respectively add 1.5 equivalents of β, β-dimethacrylic acid, 2.5 equivalents of DCC, and catalytic amount of DMAP, stir at room temperature for 2-4 hours, extract with dichloromethane, dry over anhydrous sodium sulfate, concentrate and perform column chromatography , to obtain yellow oil compound II-2 respectively. Yield 71%, 1 HNMR (300MHz, CDCl 3 ): δ=6.63(s, 1H, H Ar ), 6.52(s, 2H, H Ar ), 5.52(m, 1H, -COCH=), 5.30(s, 1H, -CH-), 5.22(s, 1H, -CH 2 CH=), 3.88(s, 6H, 2×-OCH 3 ), 3.84 (s, 3H, -OCH 3 ), 3.76 (s, 3...
Embodiment 3
[0059] Compound (s)-2-[1-(3-methyl-3-hydroxy-butyryloxy)-4-methyl-pent-3-enyl]-1,4,5,8-tetramethoxy Naphthalene (II-3), (s)-2-[1-(3-methyl-3-hydroxy-butyryloxy)-4-methyl-pent-3-enyl]-5,8- Dimethoxy-1,4-naphthoquinone (III-2) and (s)-6-[1-(3-methyl-3-hydroxy-butyryloxy)-4-methyl-penta-3 Preparation of -alkenyl]-5,8-dimethoxy-1,4-naphthoquinone (IV-3)
[0060] Dissolve (s)-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene in anhydrous dichloromethane at 0-5 ℃ were added corresponding 1.5 equivalents of β-hydroxyisovaleric acid, 2.5 equivalents of DCC, and a catalytic amount of DMAP, stirred at room temperature for 1-2 h, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated and then column chromatographed. Compound II-3 was obtained as yellow oil. Yield 62%, 1 H NMR (300MHz, CDCl 3 ): δ=6.88(s, 1H, H Ar ), 6.84(s, 2H, H Ar ), 6.09(dd, 1H, J=2.7, 5.4Hz, -CH-), 6.15(t, 1H, J=4.5Hz, -CH=), 3.93(s, 6H, 2×-OCH 3 ), 3.86(s, 3H, -OCH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com