Improved method for synthesizing ranolazine
A synthesis method and a technology of ranolazine, applied in the field of medicinal chemistry, can solve the problems of low total yield, unsuitable for industrial production, many steps, etc., and achieve the effects of reducing reaction time and production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] In order to better implement the present invention, the present invention is further described by giving examples, but the examples are not limitations of the present invention.
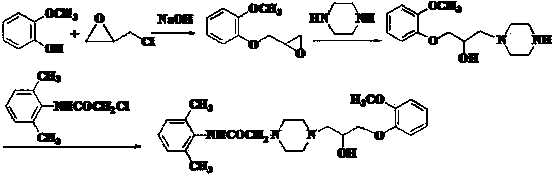
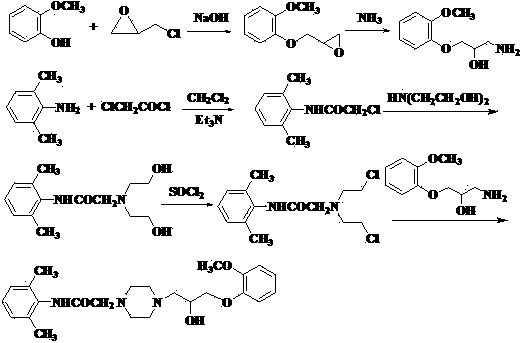
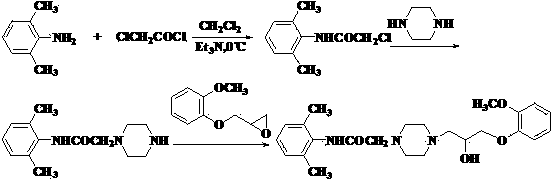
[0026] (1) Preparation of 2-(2-methylphenoxymethyl)oxirane
[0027] Add 49.6g (0.4mol) of o-methoxyphenol, 185g (2.0mol) of epichlorohydrin, 110.6g (0.4mol) of potassium carbonate, and 0.5g of benzyltriethylammonium chloride (TEBA) into a 500ml reaction bottle . Heat up to 70°C with stirring, react for 2 hours, cool to room temperature, filter, wash the filter cake with 60ml×2 epichlorohydrin, combine the filtrate, recover epichlorohydrin at 60-120°C / 50mmHg, collect 136.0-138.0°C / 30mmHg Distillation, the product 61.9g was obtained, the yield was 86.0%.
[0028] (2) Preparation of 2-chloro-N-(2,6 benzhydryl)acetamide
[0029] Put 36.4g (0.30mol) of 2,6-dimethylaniline, 600ml of dichloromethane, 31.8g (0.3mol) of sodium carbonate and 300ml of water into a 2000ml reaction bottle. Control t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com