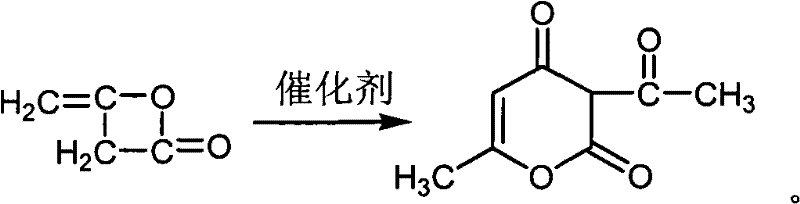
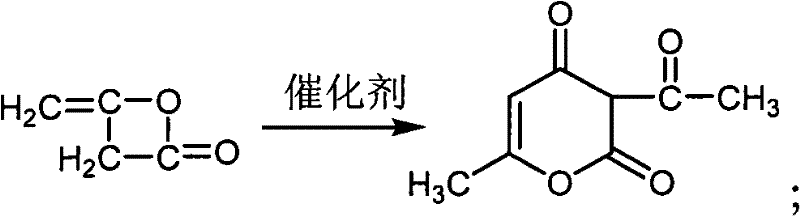
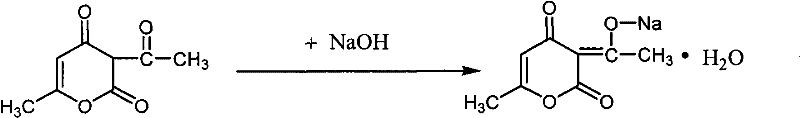Method for preparing food-grade sodium dehydroacetate
The technology of sodium dehydroacetate and dehydroacetic acid is applied in the field of preparation of food-grade sodium dehydroacetate, and can solve the problems of low purity, unstable quality, inferior color of sodium dehydroacetate, and achieve white appearance, low cost, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 270mL of ethyl acetate, 30mL of acetic anhydride and 0.2g of anhydrous sodium acetate into a 1000mL three-necked flask, and heat to 75°C while stirring. Add 200 mL of diketene into the dropping funnel, and slowly add it dropwise to the above mixture, keeping the internal temperature at about 78°C-80°C. After the dropwise addition, the reaction was continued for 2 h.
[0022] The above-mentioned completely reacted solution was transferred to a beaker, cooled to room temperature under constant stirring, and then refrigerated at 0°C for 6 hours for crystallization. Suction filtration, the filter cake was washed twice with refrigerated ethyl acetate, and dried in an oven at 75°C. 70 g of light yellow solid was obtained.
[0023] Mix the obtained 70g of solid and 200mL of absolute ethanol in a single-necked flask, stir and reflux at 80°C until the solution is clear, continue to stir for 0.5h, then transfer to a beaker for cooling and crystallization. Suction filtratio...
Embodiment 2
[0026] Add 140mL of ethyl acetate, 15mL of acetic anhydride and 0.1g of anhydrous sodium acetate into a three-necked flask, and heat to 75°C while stirring. Add 100 mL of diketene into the dropping funnel, and slowly add it dropwise to the above mixture, keeping the internal temperature at about 80°C. After the dropwise addition, the reaction was continued for 2 h.
[0027] The above-mentioned completely reacted solution was transferred to a beaker, cooled to room temperature under constant stirring, and then refrigerated at 0°C for 6 hours for crystallization. Suction filtration, the filter cake was washed twice with cold ethyl acetate, and dried in an oven at 80°C. 53 g of light yellow solid was obtained.
[0028] Mix the obtained 53g of solid and 160mL of absolute ethanol in a single-necked flask, stir and reflux at 80°C until the solution is clear, continue to stir for 0.5h, then transfer to a beaker for cooling and crystallization. Suction filtration and drying at 80°C...
Embodiment 3
[0031] Add 140mL of anhydrous toluene, 15mL of acetic anhydride and 0.1g of triethylenediamine into a three-necked flask, and heat to 75°C while stirring. Add 100 mL of diketene into the dropping funnel, and slowly add it dropwise to the above mixture, keeping the internal temperature at about 80°C. After the dropwise addition, the reaction was continued for 2 h. The above-mentioned completely reacted solution was transferred to a beaker, cooled to room temperature under constant stirring, and then refrigerated at 0°C for 6 hours for crystallization.
[0032] Suction filtration, the filter cake was washed 3 times with refrigerated toluene, and dried in an oven at 75°C. Obtained 49.3 g of light yellow solid.
[0033] The resulting 49.3g solid and 160mL absolute ethanol were mixed in a single-necked flask, stirred and refluxed at 70°C until the solution was clear, and then continued to stir for 0.5h, then transferred to a beaker for cooling and crystallization. Suction filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com