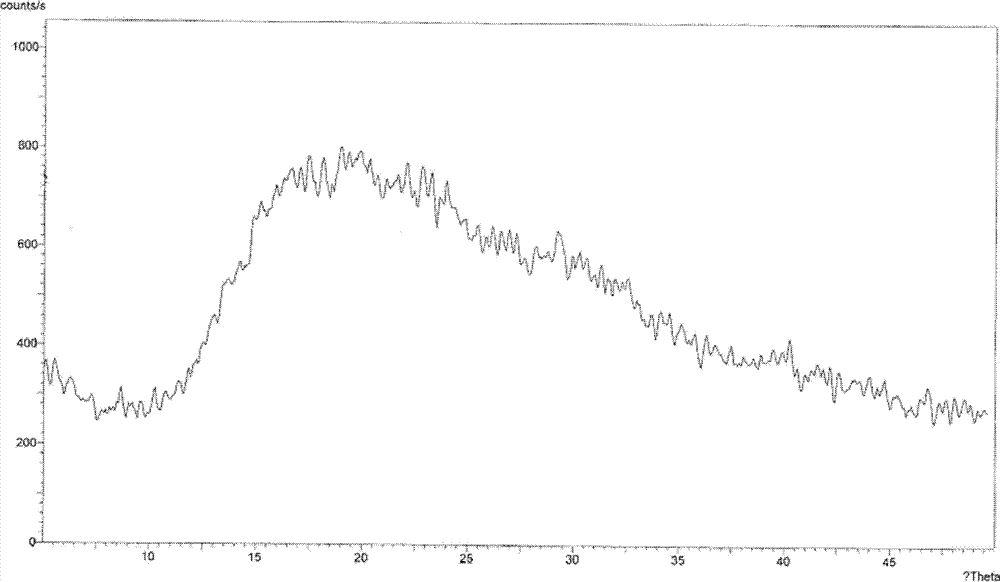
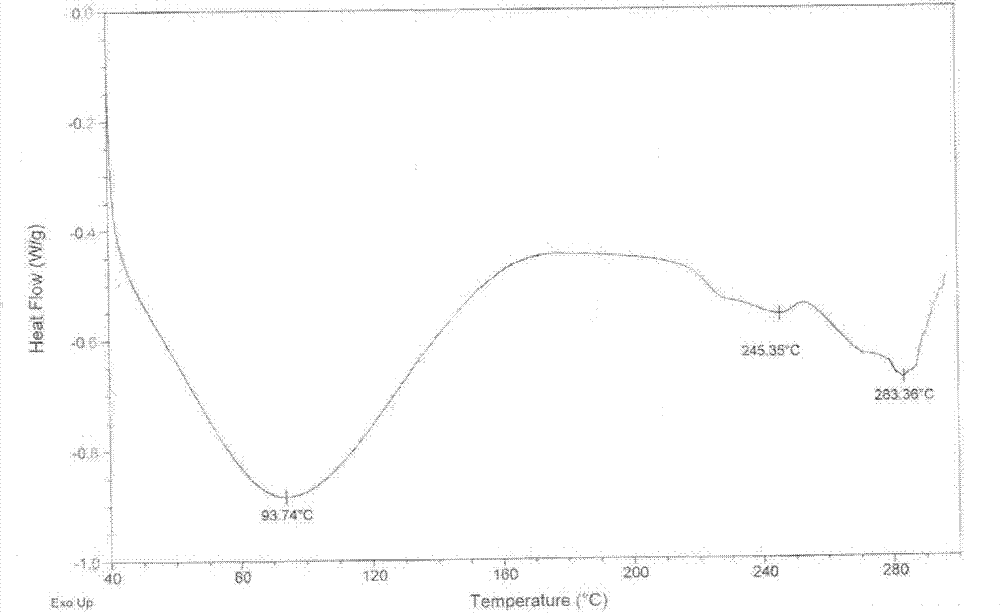
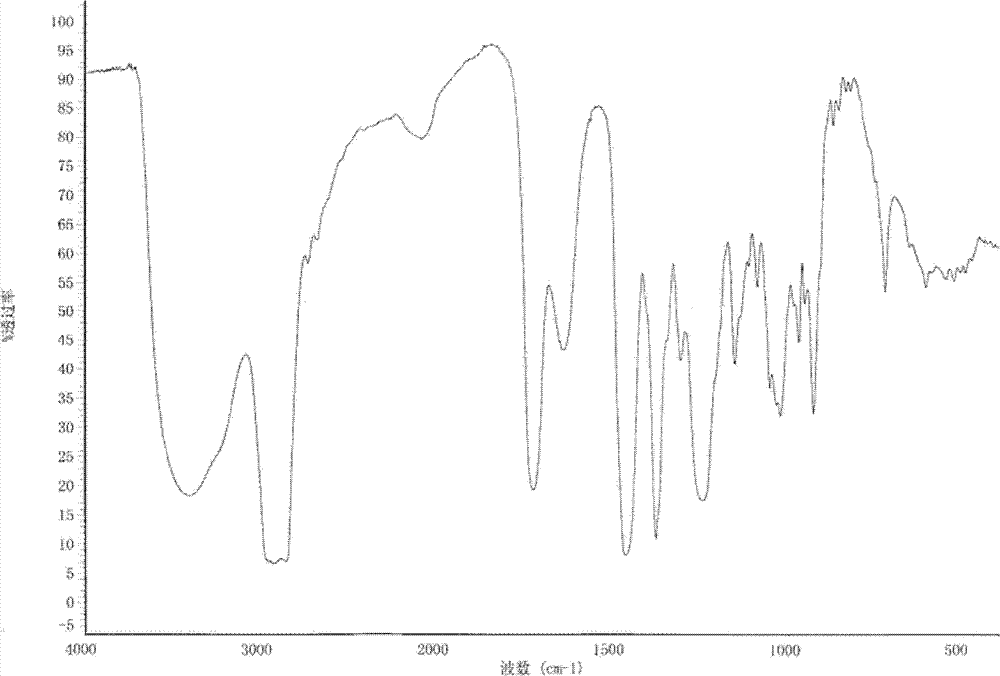
Non-sizing pipecuronium and preparation method and purpose thereof
A pipecuronium bromide and amorphous technology, which is applied in the field of amorphous pipecuronium bromide and its preparation, can solve the problems of poor resolubility of pipecuronium bromide powder injections and pain in patients, and improve resolubility and reduce Residue of organic solvents, effect of increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 pipecuronium bromide amorphous substance of the present invention
[0051] Dissolve 200 g of pipecuronium bromide in 600 ml of water with stirring, bubble and saturate with nitrogen for 2 min, and filter. The filtrate was extracted twice with 800 ml of ethyl acetate, and the organic layer was discarded to obtain an aqueous layer.
[0052] The treated aqueous layer solution was quickly frozen to -40°C, kept at a constant temperature for 4 hours, and the solution was frozen into a solid. Vacuumize the system to a vacuum degree below 20Pa, heat up gradually to -20°C, keep the temperature for 4 hours; raise the temperature to -10°C, keep the temperature for 6 hours; raise the temperature to 0°C, keep the temperature for 8 hours; naturally rise to room temperature, keep the temperature for 10 hours, That is, 185 g of the pipecuronium bromide was obtained with a yield of 92.5% and a melting point range of 267-270° C. (decomposition).
[0053]...
Embodiment 2
[0063] Embodiment 2 The preparation of pipecuronium bromide amorphous substance of the present invention
[0064] Dissolve 200 g of pipecuronium bromide in 500 ml of water with stirring, bubble and saturate with nitrogen for 2 min, and filter. The filtrate was extracted twice with 600 ml of cyclohexane, and the organic layer was discarded to obtain an aqueous layer.
[0065] The treated aqueous layer solution was quickly frozen to -40° C., kept at a constant temperature for 2 hours, and the solution was frozen into a solid. Vacuumize the system to below 18Pa, heat gradually to -20°C, keep the temperature for 4 hours; raise the temperature to -10°C, keep the temperature for 6 hours; raise the temperature to 0°C, keep the temperature for 8 hours; naturally rise to room temperature, keep the temperature for 10 hours, That is, 185 g of the pipecuronium bromide was obtained as a white solid with a yield of 92.5%, a purity of 99.8%, and a melting point range of 268-270° C. (decompo...
Embodiment 3
[0066] Embodiment 3 The preparation of pipecuronium bromide amorphous substance of the present invention
[0067] 500ml of water was saturated with nitrogen, 200g of pipecuronium bromide was added, and stirred to dissolve. Filter and extract twice with 500 ml of diethyl ether, discard the organic layer, and then saturate the aqueous layer with nitrogen for 1 minute.
[0068] The treated aqueous solution was quickly frozen to -40°C and kept at a constant temperature for 2 hours to freeze solid. The system is evacuated to below 40Pa, and the temperature is gradually raised to -20°C and kept at a constant temperature for 4 hours; the temperature is raised to -10°C and kept at a constant temperature for 6 hours; the temperature is raised to 0°C and kept at a constant temperature for 8 hours; naturally rises to room temperature and kept at a constant temperature for 8 hours, that is 188 g of the pipecuronium bromide was obtained as a white solid with a yield of 94.0%, a purity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com