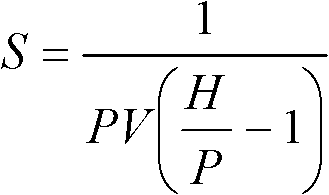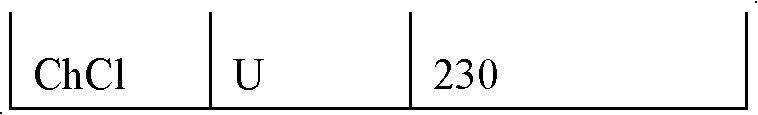Olefin selective membrane comprising an ionic liquid and a complexing agent
An ionic liquid, olefin technology, applied in membrane technology, separation method, gas treatment, etc., can solve the problems of harmful diaphragm performance, reducing olefin/paraffin selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and comparative example 1
[0044] Synthetic choline chloride-urea ionic liquid (embodiment 1)
[0045] A certain amount of pure (99 wt%) choline chloride was added to the flask. An amount of pure (99 wt%) urea was also added to the flask such that the molar ratio of choline chloride to urea was 1:2. The mixture was stirred at 250-500 revolutions per minute (rpm) at 80°C until a homogeneous liquid formed (this was after about 1 hour). A quantity of silver chloride (AgCl) is added and dissolved to the ionic liquid until a point of saturation is approached or reached. This composition is hereinafter referred to as ChCl:U2AgCl.
[0046] Synthesis of 1-butyl-3-methylimidazole Two (trifluoromethane) sulfonamide ionic liquid (comparative example 1)
[0047] A certain amount of ionic liquid 1-butyl-3-methylimidazole Chloride (more than 95 wt%) was added to a 1-neck round bottom flask on a stir plate. Deionized water was added to the ionic liquid (5:1 weight / weight (w / w)) to dissolve the ionic liquid the...
preparation Embodiment 1 and comparative example 1
[0049] Prepare the diaphragm of Example 1 and Comparative Example 1
[0050] 0.5 to 2 g of Example 1 ionic liquid (comprising silver chloride as metal salt favoring olefins) was placed on a glass fiber sample supported by wax paper, and a similar amount of Comparative Example 1 ionic liquid was placed on another Fiberglass samples supported on waxed paper. Each sample was loaded into a permeation cell, and each permeation cell was secured into a pure gas permeation system. Osmotic systems are constant volume / variable pressure systems routinely used in the art. Both samples were exposed to vacuum at 70°C for at least 16 hours prior to testing.
[0051] Test matrix olefin adsorption properties
[0052] Samples of each septum matrix (5 ml each) were placed in vials in a parallel pressure reactor (PPR). The samples were exposed to 200 psi (1379 kPa) ethylene at 30°C. The pressure of ethylene was maintained at 200 psi (1379 kPa) by the PPR for the duration of each test. The a...
Embodiment 2-3 and comparative example 2-7
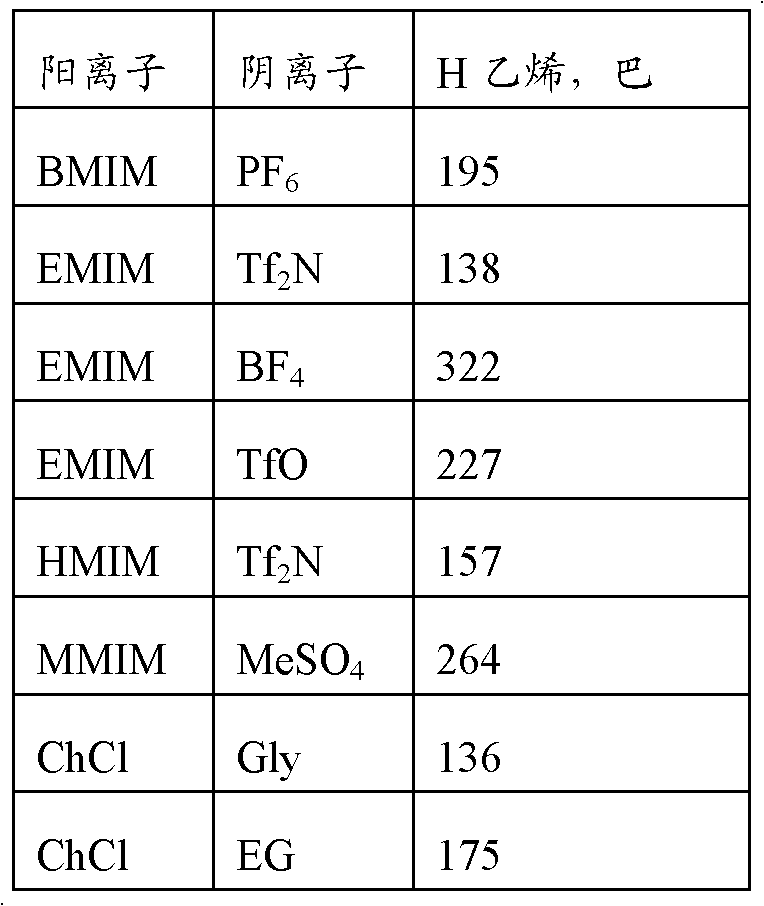
[0058] Using the methods of Example 1 and Comparative Example 1 respectively, two additional example compositions (Example 2-3) and six comparative compositions (Comparative Examples 2-7) were used to prepare separators, the composition of which is shown in Table 2.
[0059] Table 2
[0060]
[0061] Key: ChCl is Choline Chloride
[0062] U is urea
[0063] Gly is glycerin
[0064] EG is ethylene glycol
[0065] * 2 represents stoichiometry, ie, 2 moles of urea, glycerol, or EG per mole of choline chloride.
[0066] BMIM[AOT] is 1-butyl-3-methylimidazole Dioctyl sulfosuccinate
[0067] BMIM[Tf2N] is 1-butyl-3-methylimidazole Bis(trifluoromethane)sulfonimide
[0068] BMIM[BF4] is 1-butyl-3-methylimidazole Tetrafluoroborate
[0069] It should be noted that although Comparative Examples 2-4 meet the adsorption requirements for the ionic liquids of the present invention operating at 30 °C membrane, they do not contain metal salts that can contribute to olefins, whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com