Anti-insect protein Cry1A.401 and expression vector and application thereof
A technology of insect-resistant protein and carrier, which is applied in the field of genetic engineering and biological control, can solve problems such as ecological imbalance, environmental pollution, and lack of insect-resistant varieties, and achieve the effects of reducing usage, reducing environmental pollution, and expanding insect-resistant spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of Cry1A.401 gene
[0033] Through codon optimization, Cry1Ab, Cry1F and Cry1Ac were synthesized (the synthesis work was completed by Sangon Bioengineering (Shanghai) Co., Ltd.), and then by the SOE method (1992, Cai Yuyang), the first and second functional domains of Cry1Ab, The third functional domain of Cry1F and the fragment of Cry1Ac (1837bp to 3531bp) are spliced together to form a new Cry1A.401 DNA sequence, the nucleotide sequence of which is shown in SEQ ID NO.1. It is generally believed that: Bt protein is composed of three structural domains, domain I includes 250-300 amino acids at the N-terminal of the active insecticidal protein, and consists of 7 α-helices, one of which is relatively hydrophobic and located in the structural domain The center of I is surrounded by the other 6 α-helices. It has been proven that it is related to the toxicity of insecticidal proteins. The hydrophobic α-helix has the ability to insert into the midgut ce...
Embodiment 2
[0034] Example 2 Expression of Cry1A.401 gene in prokaryotic system and detection of product toxicity
[0035] In order to detect the in vitro expression of the modified Cry1A.401 gene and its toxicity to the corn borer, we constructed a Bt prokaryotic expression vector. According to the needs of cloning the Bt gene, the NdeI endonuclease recognition site sequence AAGGAGATATACATA was added to the 5' end of the primer sequence, and the XhoI endonuclease recognition site sequence GGTGGTGGTGCTCGAG was added to the 3' end. The designed primer sequences are listed as: F: AAGGAGATATACATA TGGACAACAACCCGAAC; R: GGTGGTGGTGCTCGAG TTCTC CATAAGGAGTAA. Using the spliced DNA as a template, the Cry1A.401 gene was amplified with the adapter-added primers, and the Cry1A.401 gene fragment was recovered and purified by the gel recovery kit. Digest pET30a with restriction endonucleases NdeI and XhoI, recover and purify. The two fragments were ligated, and the resulting prokaryotic express...
Embodiment 3
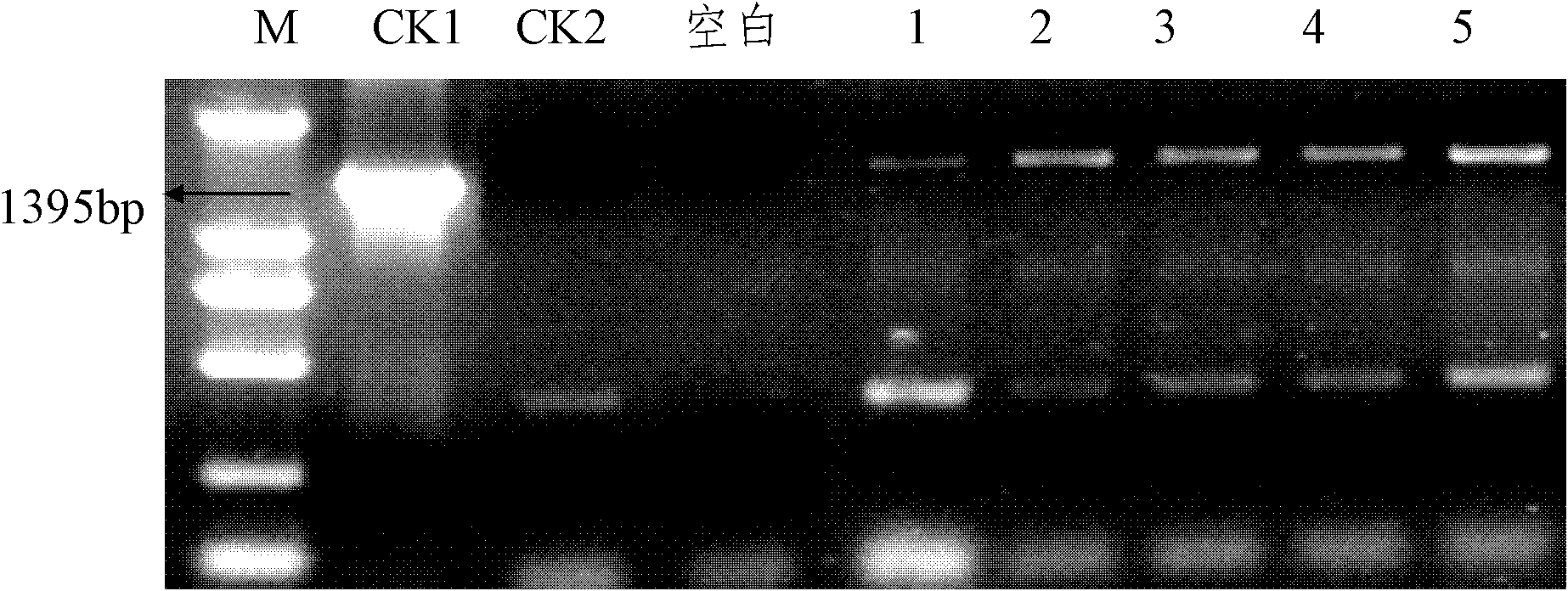
[0048] Example 3 Expression of Cry1A.401 Gene in Transgenic Plants and Toxicity Detection of Expression Products
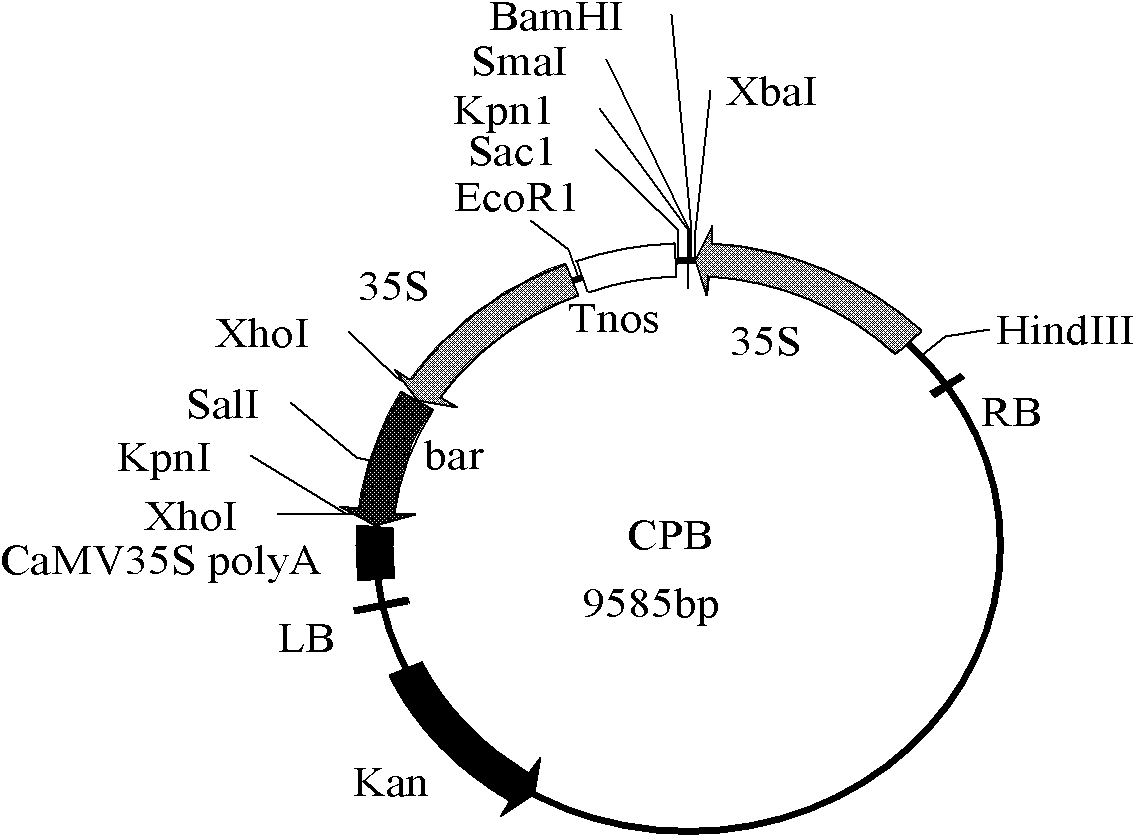
[0049] Add the SmaI endonuclease recognition site sequence CTCTAGAGGATCCCC and TCGAGCTCGGTACCC at the 5' and 3' ends of the primer sequence respectively, and design the primer as: F: 5' CTCTAGAGGATCCCC ATGGACAACAACCCGAAC3';R:5' TCGAGCTCGGTACCC CTACTCGAGTGTGGCAGTAAC 3', using the synthesized Cry1A.401 nucleotide sequence as a template, amplified with primers with adapters, and recovered the target fragment with a gel recovery purification kit. At the same time, the plant expression vector CPB was digested with SmaI, and the CPB fragment after digestion was recovered and purified. The two fragments were ligated (In-Fusion HD cloning kit, Clontech), and the eukaryotic expression plasmid CPB-Cry1A.401 (full text unified) was constructed (the promoter was CaMV35S, and the marker gene was the herbicide-resistant gene Bar of Streptomyces hygroscopicus ). Detection b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com