Application and preparation method of compound madopar to preparation of information therapeutic medicine and novel medicine prepared from compound madopar
A chemotherapeutic drug, a technology for treating drugs, applied in the field of drugs, can solve problems such as nausea and vomiting, orthostatic hypotension, head, face, tongue, upper limbs and upper parts of the body, cannot be used twice, and limit treatment effects, so as to avoid toxic effects. Side effects, increased use value, effect of overcoming side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
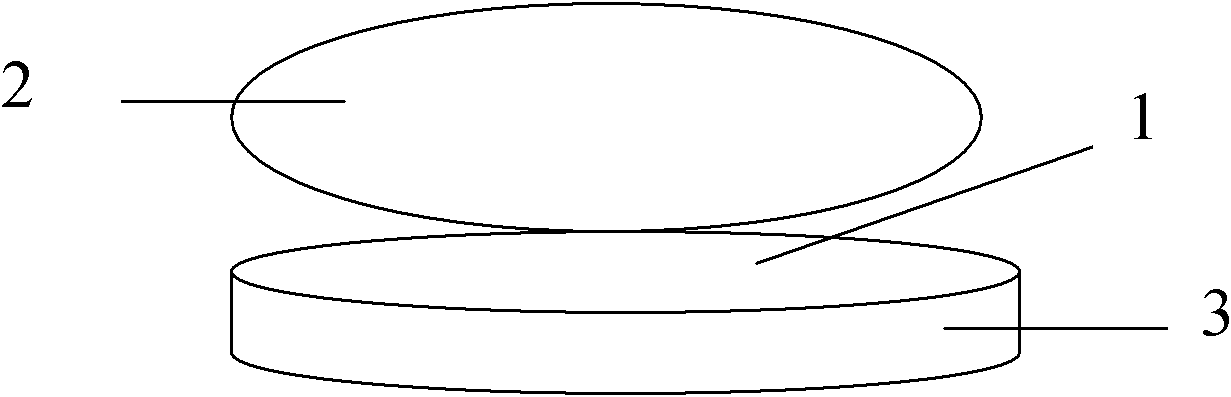
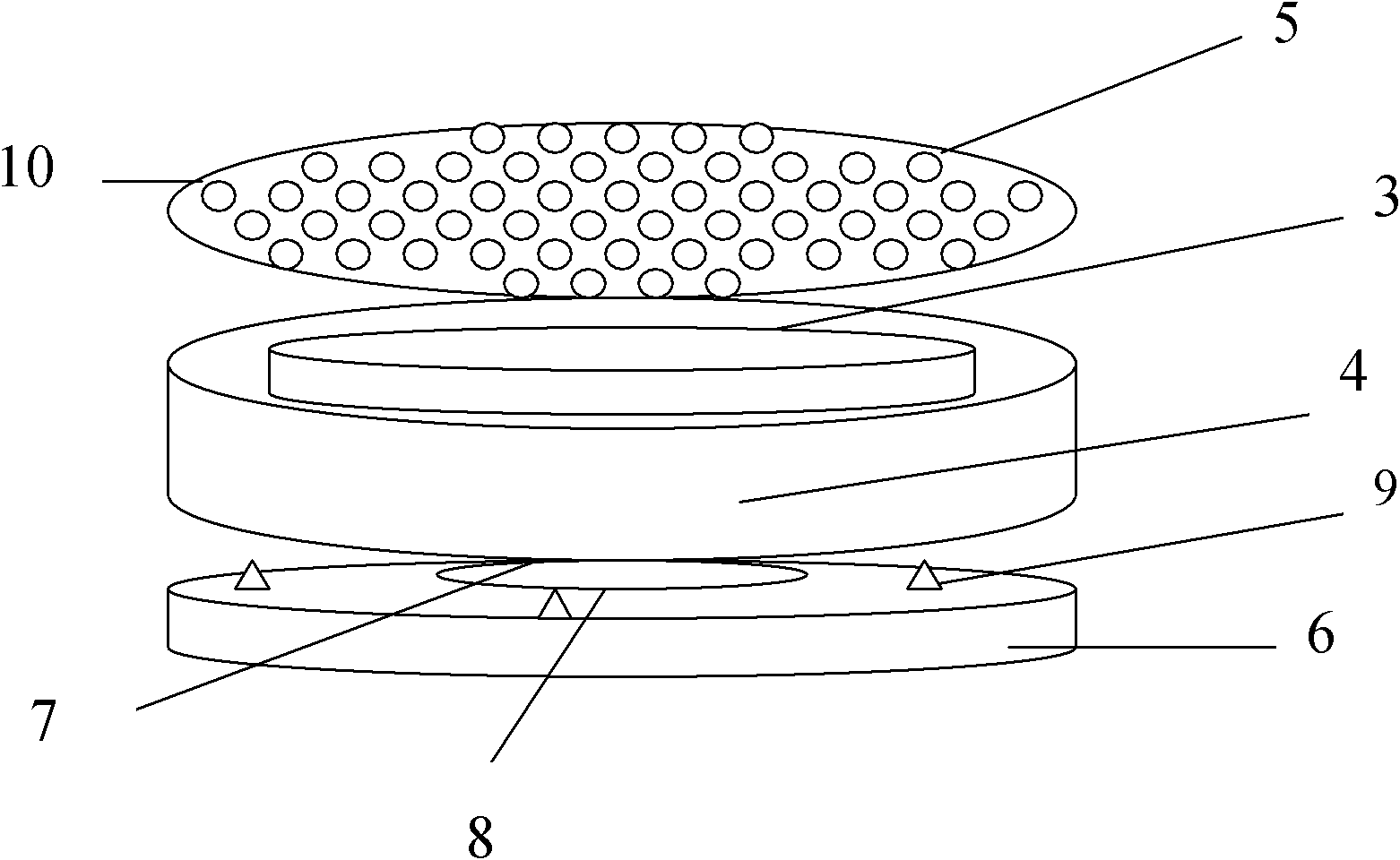
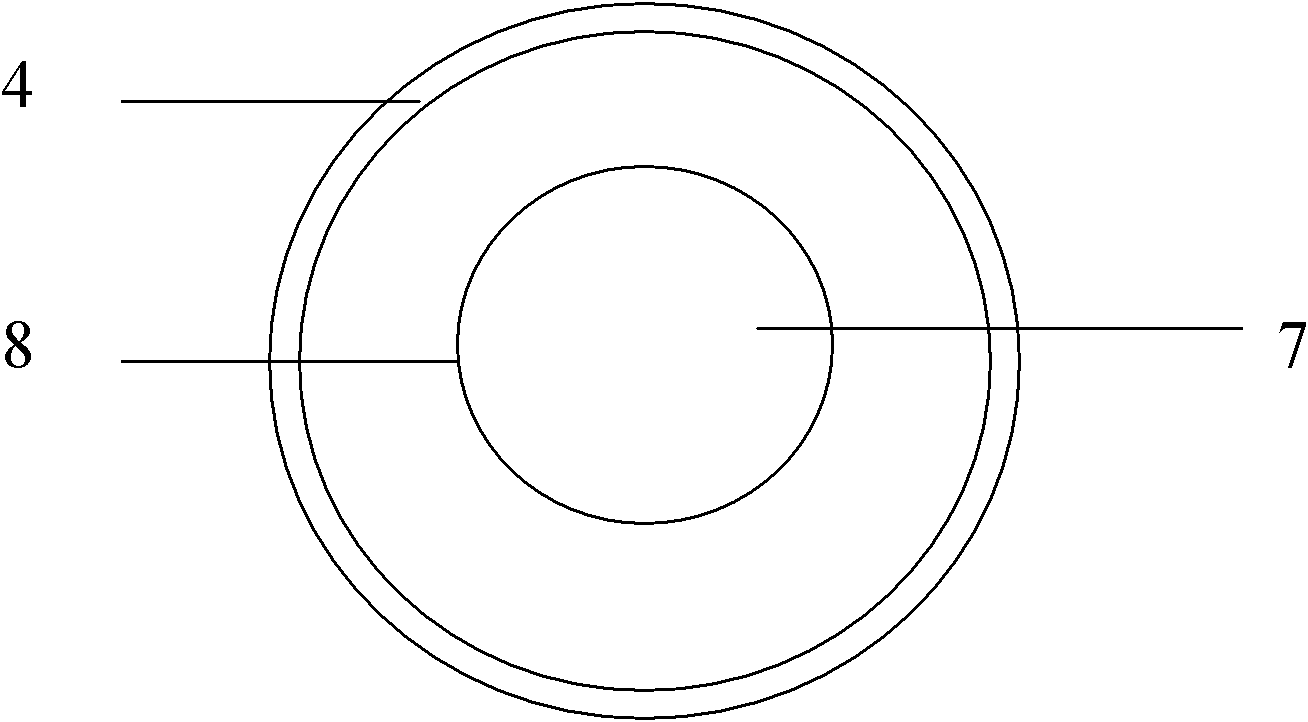
[0044] Weigh Madopar raw material (1) according to the following weight: 900 grams of Madopar raw material (1) (content ≥ 99%), put raw material (1) into the filling machine, and use a microporous air-permeable paper bag to divide according to the net weight of each bag of 30 grams. Pack into 30 bags, heat-melt and seal, and make Madopa drug information stuffing bag (3). Simultaneously, make magnetic suspension (card) (8) container (4) box 30 in addition. The manufacturing method of the magnetic suspension (card) (8) container (4) box is: use plastic mold injection molding to produce an oblate container (4) box with an inner hollow diameter of 5-10cm and a height of 0.5-1.5cm, and the container (4) box A number of holes (5) with a diameter of 0.1 cm are placed on the outside of the lower part, and a magnetic sheet (7) with a magnetic force of 500-1500 Gauss is placed on the concave surface of the top of the container (4), and a magnetic sheet (7) of the same size is added on t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com