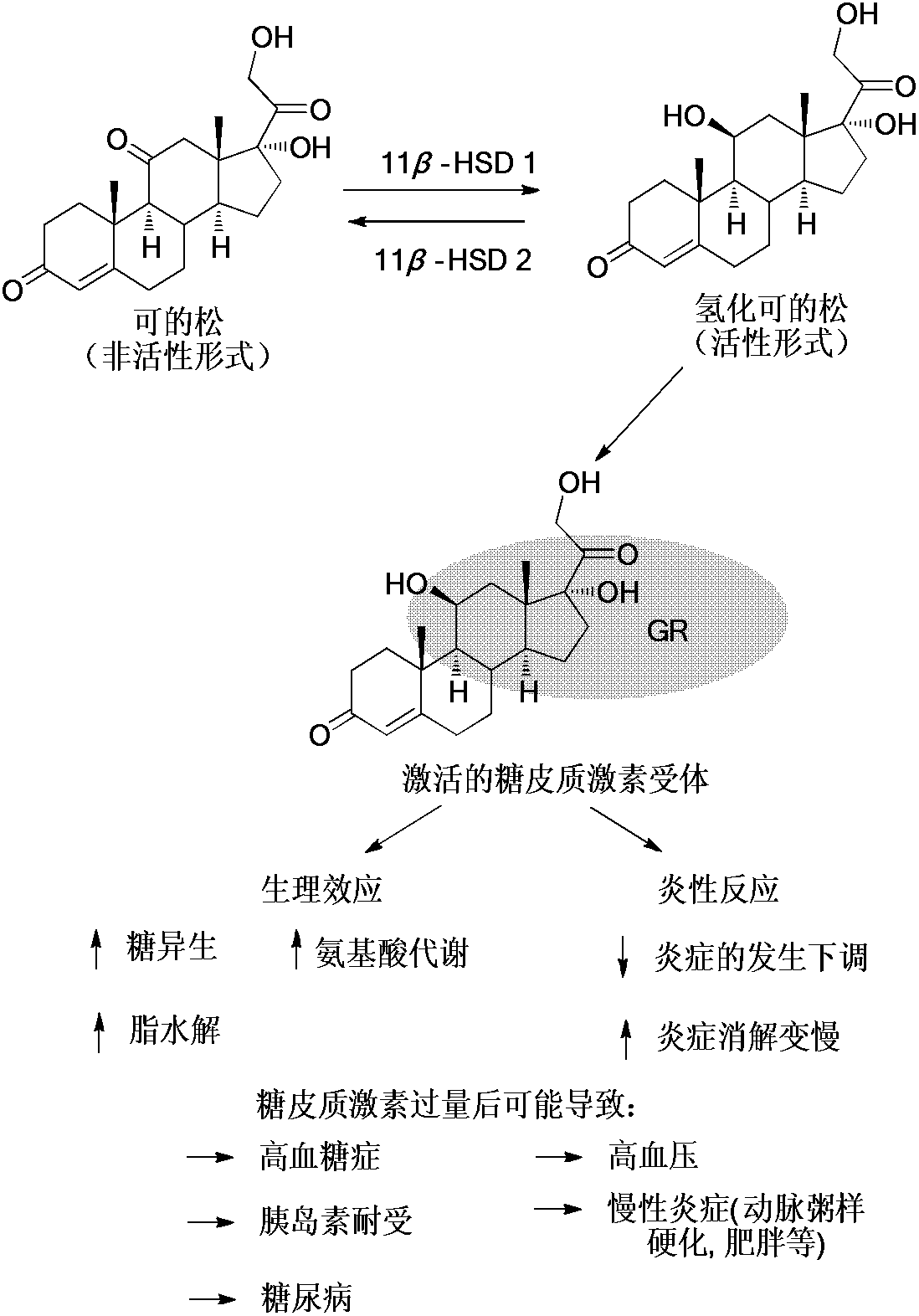
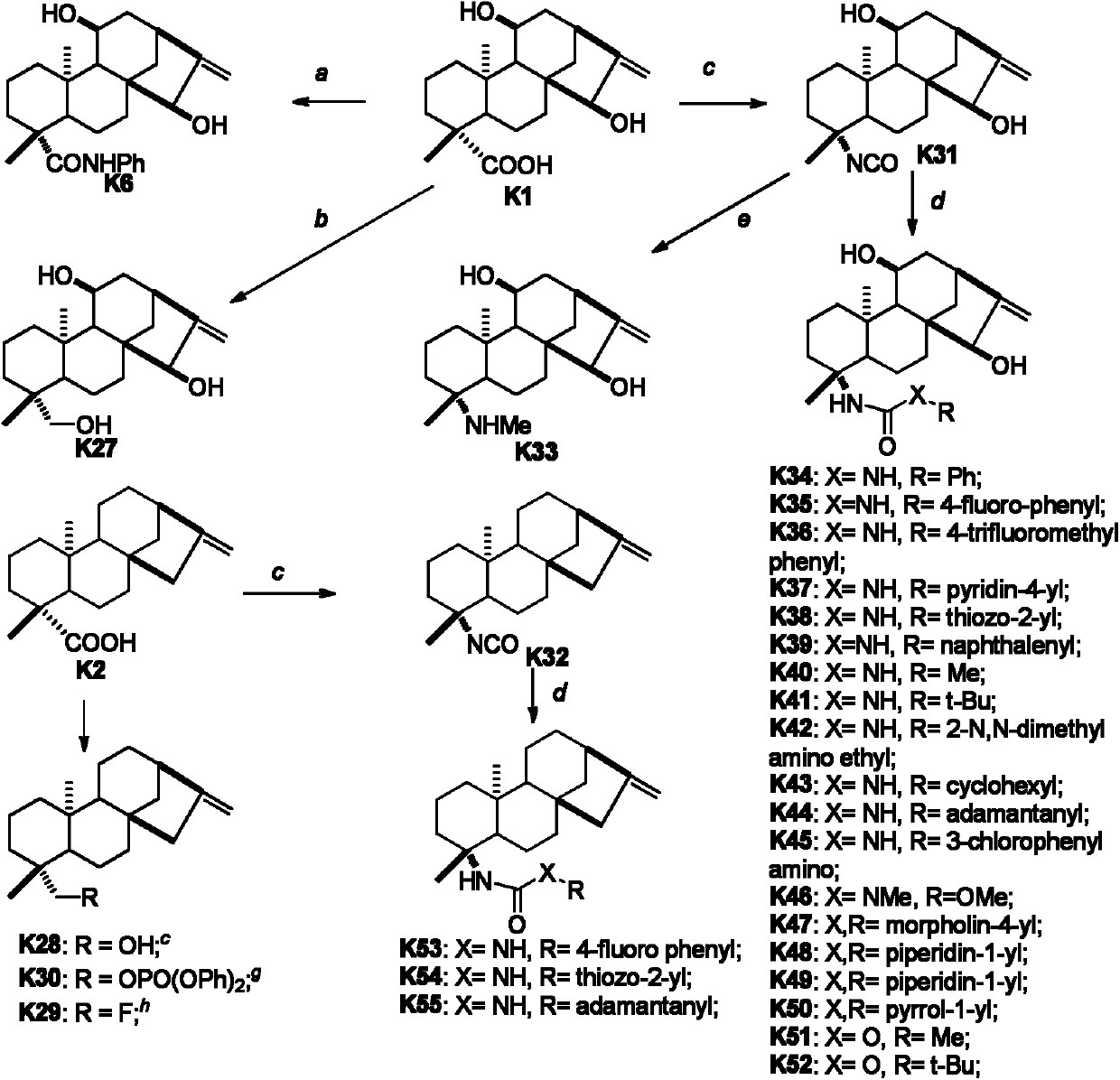
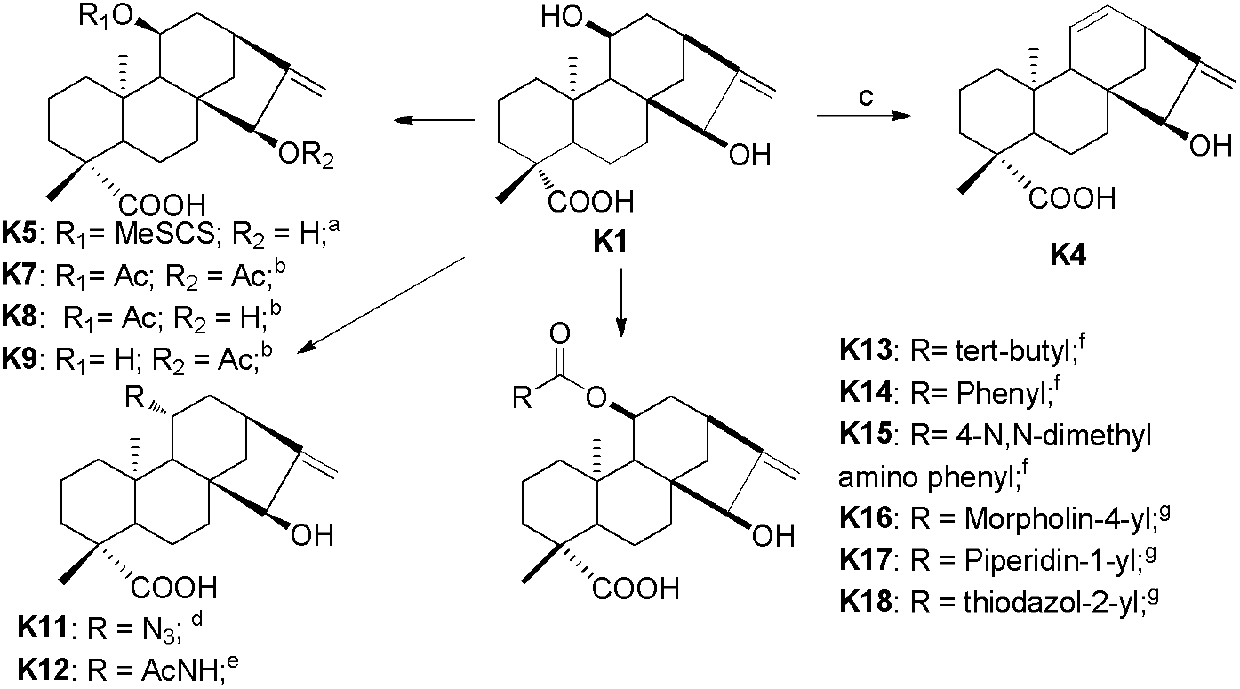
Kaurene diterpene derivative, medicinal composition thereof and application of kaurene diterpene derivative to medicament
A technology of kaurane-type and derivatives, which is applied in the field of kaurene-type diterpene derivatives, and can solve problems such as the limitation of clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of compound K1:
[0051]Dried plant chrysanthemum (Nonelia insignis Franech) branches and leaves 22kg, after crushing, use 95% ethanol to extract three times (20L / time, 2 days / time), extract with ethyl acetate after concentrating, obtain crude product ( 750g), the crude product (200g) was segmented with silica gel (1000g) mixing column chromatography (sequentially with petroleum ether / acetone (9.5:0.5), acetone, methanol elution), wherein the crude product (113g) of the large polar section was repeatedly used Compound K1 (65 g) was obtained by normal and reverse phase column chromatography.
Embodiment 2
[0053] Preparation of compounds A, B, C, K2:
[0054] Dried plant Dalibai (Diplopterygium gigantean (wall.) Nakai) aerial part 5.4kg, crushed and extracted three times (15L / time) with 95% ethanol, the extract was suspended in water, then extracted four times with ethyl acetate, the organic phase After concentrating, it was dissolved with methanol, decolorized by MCI column, and then the extract was mixed with silica gel and column chromatography was segmented, and the small polar segment was repeatedly used for positive and negative phase column chromatography to obtain compounds A (450mg), B (18mg), and C (14mg) , K2 (3g).
Embodiment 3
[0056] Preparation of Compound K3
[0057]
[0058] Under ice-water bath condition, in the DCM (1.5mL) solution of compound K1 (33mg), add PDC (58mg), make it rise to room temperature naturally after reacting for 2 hours, TLC finds that the reaction is complete, and the reaction solution is directly column chromatographed ( Petroleum ether:ethyl acetate=3:1) to obtain compound K3 (31 mg, 95%).
[0059] Compound K3: white solid, 1 H NMR (500MHz, MeOD) δ6.03(s, 1H), 5.42(s, 1H), 3.28(s, 1H), 2.82(d, J=12Hz, 1H), 2.65(dd, J=12Hz, 4Hz , 1H), 2.57(d, J=12Hz, 1H), 2.20(d, J=8Hz, 1H), 2.02(m, 1H), 1.97(d, J=12Hz, 1H), 1.92(s, 1H) , 1.89(d, J=4Hz, 1H), 1.88(s, 1H), 1.80(d, J=8Hz, 1H), 1.70-1.76(m, 2H), 1.59(s, 1H), 1.57(d, J=4Hz, 1H), 1.48(d, J=8Hz, 1H), 1.29(s, 3H), 1.25(d, J=4Hz, 4H), 1.23(dd, J=12Hz, 4Hz, 2H), 1.09 (dd, J=12Hz, 4Hz, 1H), 1.02(s, 3H), 0.88(t, J=8Hz, 4Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com