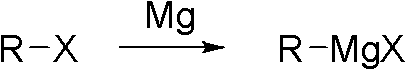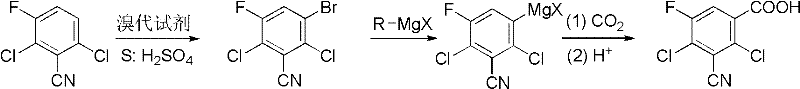Preparation method of 2,4-dichloro-3-cyano-5-fluobenzoic acid
A technology of fluorobenzoic acid and cyano, which is applied in the field of preparation 2, can solve the problems of potential safety hazards and low yield of Sandmeyer reaction, and achieve the effects of low product cost, short steps and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of 2,6-dichloro-3-cyano-5-fluorobromobenzene
[0021] Add 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 42.72g (0.24mol) of N-bromosuccinimide to 100mL of concentrated sulfuric acid, stir at room temperature for 30h, and pour the reaction solution into In 200mL of ice-water mixture, a white solid precipitated out, filtered with suction, washed the filter cake with water, and dried to obtain 52.8g of a white solid product, which was 2,6-dichloro-3-cyano-5-fluorobromobenzene, with a yield of 98% . m.p.122-124°C; 1 H NMR (400MHz, CDCl 3 )δ: 7.69 (d, J=7.6Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ: 112.1(d, J=3Hz), 116.5, 122.1(d, J=9Hz), 125.1(d, J=20Hz), 125.2(d, J=24Hz), 134.0(d, J=4Hz), 156.0 (d, J=254Hz); EI-MS (m / z): 269 (M + ).
Embodiment 2
[0023] Synthesis of 2,6-dichloro-3-cyano-5-fluorobromobenzene
[0024] Add 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 34.44g (0.12mol) of dibromocyanuric acid to 100mL of concentrated sulfuric acid, stir and react at 0°C for 40h, and pour the reaction solution into 200mL In the ice-water mixture, a white solid was precipitated, filtered with suction, washed the filter cake with water, and dried to obtain 52.8 g of a white solid product, 2,6-dichloro-3-cyano-5-fluorobromobenzene, with a yield of 96%, n.p. 122.5-124°C.
Embodiment 3
[0026] Synthesis of 2,6-dichloro-3-cyano-5-fluorobromobenzene
[0027] Add 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 34.2g (0.12mol) of bromobarbituric acid to 100mL of concentrated sulfuric acid, stir and react at room temperature at 50°C for 5h, and pour the reaction solution into 200mL In the mixture of ice and water, a white solid precipitated out. Suction filtration, washing the filter cake with water, and drying gave 50.6 g of a white solid product, and the yield of 2,6-dichloro-3-cyano-5-fluorobromobenzene was 94%. n.p.122-124℃
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com