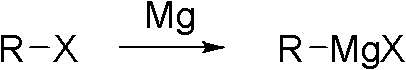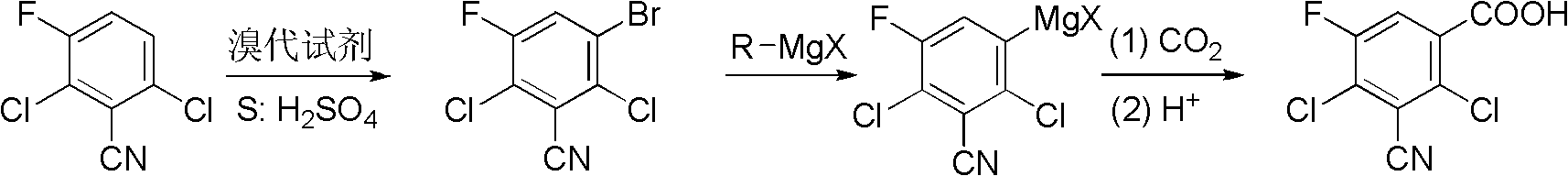Preparation method of 2,4-dichloro-3-cyano-5-fluobenzoic acid
A technology of fluorobenzoic acid and cyano group, applied in the field of preparation 2, can solve the problems of potential safety hazard, low Sandmeier reaction yield, etc., and achieve the effects of low product cost, short steps, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of 2,6-Dichloro-3-cyano-5-fluorobromobenzene
[0021] 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 42.72g (0.24mol) of N-bromosuccinimide were added to 100mL of concentrated sulfuric acid, the reaction was stirred at room temperature for 30h, and the reaction solution was slowly poured into In 200 mL of ice-water mixture, white solid was precipitated, suction filtered, the filter cake was washed with water, and dried to obtain 52.8 g of white solid product, which was 2,6-dichloro-3-cyano-5-fluorobromobenzene, and the yield was 98%. . m.p.122-124℃; 1 H NMR (400MHz, CDCl 3 )δ: 7.69 (d, J=7.6Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ: 112.1(d, J=3Hz), 116.5, 122.1(d, J=9Hz), 125.1(d, J=20Hz), 125.2(d, J=24Hz), 134.0(d, J=4Hz), 156.0 (d, J=254 Hz); EI-MS (m / z): 269 (M + ).
Embodiment 2
[0023] Synthesis of 2,6-Dichloro-3-cyano-5-fluorobromobenzene
[0024] 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 34.44g (0.12mol) of dibromocyanuric acid were added to 100mL of concentrated sulfuric acid, the reaction was stirred at 0°C for 40h, and the reaction solution was slowly poured into 200mL In the ice-water mixture, a white solid was precipitated, suction filtered, the filter cake was washed with water, and dried to obtain 52.8 g of a white solid product, 2,6-dichloro-3-cyano-5-fluorobromobenzene, the yield was 96%, n.p. 122.5-124℃.
Embodiment 3
[0026] Synthesis of 2,6-Dichloro-3-cyano-5-fluorobromobenzene
[0027] 38g (0.2mol) of 2,6-dichloro-3-fluorobenzonitrile and 34.2g (0.12mol) of bromobarbituric acid were added to 100mL of concentrated sulfuric acid, the reaction was stirred at 50°C at room temperature for 5h, and the reaction solution was slowly poured into 200mL In the ice-water mixture, a white solid was precipitated, suction filtered, the filter cake was washed with water, and dried to obtain 50.6 g of a white solid product. The yield of 2,6-dichloro-3-cyano-5-fluorobromobenzene was 94%. n.p.122-124℃
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com