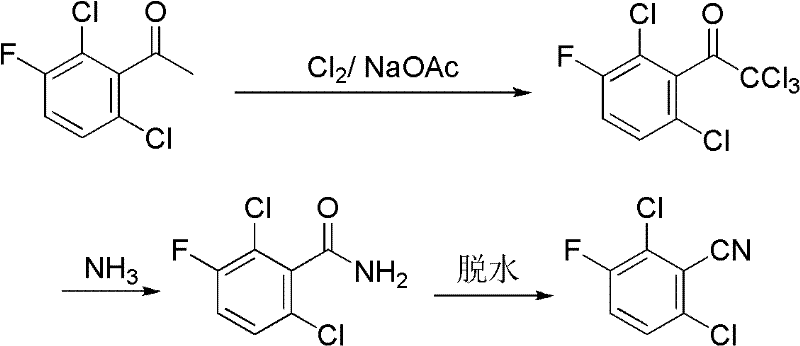
Preparation method of 2,6-dichloro-3-fluorobenzonitrile
A technology of fluorobenzonitrile and fluoroacetophenone, which is applied in the field of preparation 2, can solve the problems of low total yield, many reaction steps, and high product cost, and achieve the effects of high product quality yield, low production cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of α,α,α,2,6-pentachloro-3-fluoroacetophenone
[0028] In a 500mL round bottom flask equipped with mechanical stirring, reflux condenser, and tail gas absorption device, add 82.8g (0.4mol) of 2,6-dichloro-3-fluoroacetophenone, 200mL of glacial acetic acid, and heat up to 70°C. Chlorine gas was introduced to react for 3 hours, and TLC monitored that 2,6-dichloro-3-fluoroacetophenone was completely converted into α,α,2,6-tetrachloro-3-fluoroacetophenone, and anhydrous sodium acetate was added to the system 50g, continue to react with chlorine at 70°C for 1h, TLC detects that the reaction is complete, after cooling to room temperature, blow in compressed air to drive away excess chlorine in the system, filter to remove the solid sodium chloride generated in the reaction, recover the solvent acetic acid, add 200ml acetic acid Dissolved in ethyl ester, washed with water, anhydrous Na 2 SO 4 After drying, the ethyl acetate solvent was recovered to obtain 121.8 g o...
Embodiment 2
[0030] Synthesis of α,α,α,2,6-pentachloro-3-fluoroacetophenone
[0031] In a 500mL round bottom flask equipped with mechanical stirring, reflux condenser, and tail gas absorption device, add 82.8g (0.4mol) of 2,6-dichloro-3-fluoroacetophenone, 200mL of glacial acetic acid, and heat up to 90°C. Chlorine gas was introduced to react for 2 hours, and TLC monitored that 2,6-dichloro-3-fluoroacetophenone was completely converted into α,α,2,6-tetrachloro-3-fluoroacetophenone, and anhydrous sodium acetate was added to the system 50g, continue to react with chlorine at 90°C for 1h, TLC detects that the reaction is complete, after cooling to room temperature, blow in compressed air to drive away excess chlorine in the system, filter to remove the solid sodium chloride generated in the reaction, recover the solvent acetic acid, add 200mL toluene dissolved, washed with water, anhydrous Na 2 SO 4 After drying, the toluene solvent was recovered to obtain 120.4 g of a colorless oily liquid...
Embodiment 3
[0033] Synthesis of 2,6-dichloro-3-fluorobenzamide
[0034] In a 1000mL round bottom flask equipped with mechanical stirring and tail gas absorption device, add α,α,α,2,6-pentachloro-3-fluoroacetophenone 124.2g (0.4mol), 400mL ethanol, keep the system at 20°C Continue the stirring reaction below 20° C. for 3 h after passing ammonia gas to saturation, and recover the solvent and the chloroform generated by the reaction under reduced pressure to obtain 81.5 g of 2,6-dichloro-3-fluorobenzamide as a solid (98% yield). Melting point 183-185°C, HPLC content analysis 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com