Preparation method of chiral five-membered ring sulfite with active functional groups on alpha-carbon in substituent
A technology of cyclic sulfites and active functional groups is applied in the field of preparation of chiral five-membered cyclic sulfites, which can solve problems such as environmental pollution, dehydration, troublesome separation, etc., and achieves high regioselectivity, high product yield, and operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
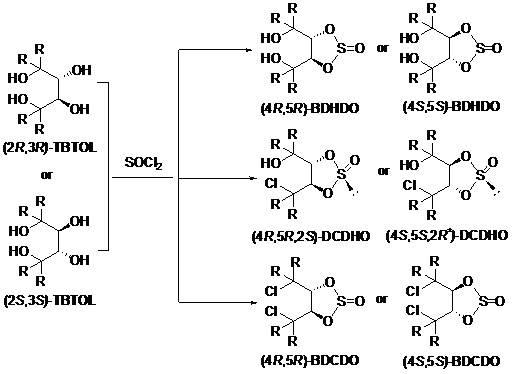
[0019] Cooled in an ice bath (2 R ,3 R 0.426 g (1 mmol) of )-1,1,4,4-tetraphenylbutanetetraol and 0.3 mL of pyridine were added to a mixture of 6 mL of tetrahydrofuran and 0.1 mL of thionyl chloride was added, followed by stirring for 2 hours. The resultant was treated with water, the organic layer was separated, the aqueous phase was extracted with ether, the extracts were combined, dried over anhydrous sodium sulfate, and 0.496 g of (4 R ,5 R )-4,5-bis(diphenylhydroxymethyl)cyclosulfite, m.p. 165-167 o C. [α] D 20 = +65.8 ( c 0.19, CHCl 3 ). 1 H-NMR (300 MHz, CDCl 3 ): δ 7.50 (d, J =7.5 Hz, 2H), 7.42 (d, J =7.2 Hz, 2H), 7.34-7.19 (m, 10H), 7.03 (s, 10H), 5.97 (d, J =2.1 Hz, 1H), 5.90 (d, J =2.1 Hz, 1H), 4.49 (s, 1H, disappears after adding water), 3.70 (t, J =6.0 Hz, 4H), 2.37 (s, 1H, disappears after adding water), 1.83 (t, J =6.0 Hz, 4H). 13 C-NMR (75 MHz, CDCl 3 ): δ 145.1, 143.3, 141.5, 141.0, 128.7, 128.5, 128.4, 128.0, 127.4, 127.1, 126.9, 125.9, 89....
Embodiment 2
[0022] According to the same operation as in Example 1, (4 S ,5 S )-4,5-bis(diphenylhydroxymethyl)cyclosulfite was obtained in 79% yield, m.p. 165-168 o C. [α] D 20 = -66.0 ( c 0.30, CHCl 3 ). 1 H NMR (300 MHz, CDCl 3 ): δ 7.50 (d, J =7.5 Hz, 2H), 7.43 (d, J =7.2 Hz, 2H), 7.35-7.13 (m, 8H), 7.04 (s, 8H), 5.97 (d, J =2.1 Hz, 1H), 5.91 (d, J =2.1 Hz, 1H), 4.48 (s, 1H, disappears after adding water), 3.75 (t, J =6.3 Hz, 4H, -CH 2 O of THF), 2.12 (s, 1H, disappears after adding weighted water), 1.85 (t, J =6.3 Hz, 4H, -CH 2 O of THF). 13 C NMR (CDCl 3 , 75 MHz): δ 144.7, 142.8, 141.1, 140.5, 128.4, 128.2, 128.0, 127.7, 127.4, 127.1, 126.7, 126.3, 125.7, 125.5, 89.3, 56.7, 678.4,
Embodiment 3
[0024] Cooled in an ice bath (2 R ,3 R 0.426 g (1 mmol) of )-1,1,4,4-tetraphenylbutanetetraol and 0.3 mL of pyridine were added to a mixture of 6 mL of dichloromethane and 0.1 mL of thionyl chloride was added, followed by stirring for 2 hours. The resultant was treated with water, the organic layer was separated, the aqueous phase was extracted with ether, the extracts were combined, dried over anhydrous sodium sulfate, and the solvent-free (4 R ,5 R )-4,5-bis(benzhydrylol)cyclosulfite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com