Synthesis method of cyclopropyl allene derivatives
A technology of cyclopropyl allene and synthesis method, which is applied in the direction of organic chemistry methods, chemical instruments and methods, hydrocarbons, etc., and can solve the problem of difficulty in synthesizing cyclopropyl allene derivatives, expensive terminal alkynes, terminal alkyne Limited types of hydrocarbons, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
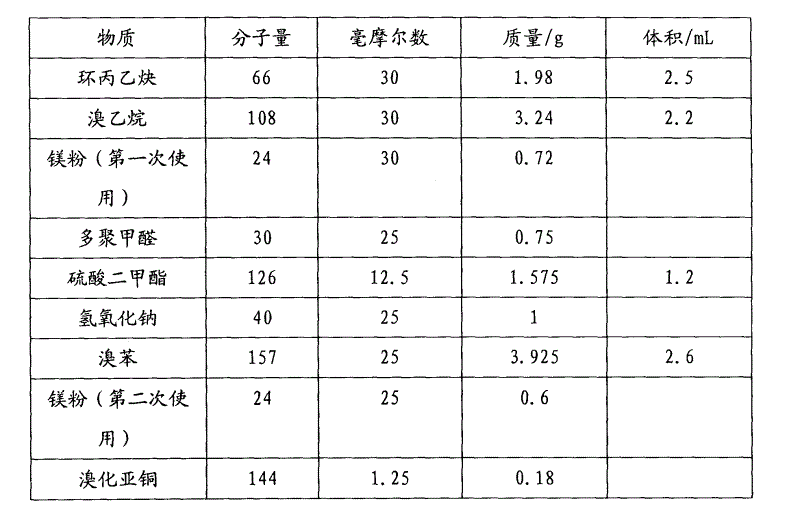
[0033] The specific feeding table of this embodiment is as figure 1 shown.
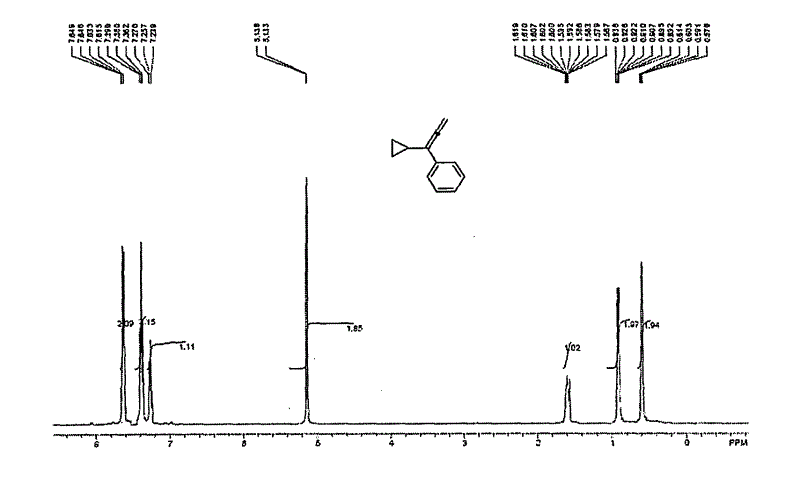
[0034] Add 0.72g of magnesium powder, a grain of iodine and a stirring bar into a 50mL round bottom flask, vacuumize at room temperature (20°C), blow nitrogen, add 30mL of anhydrous ether, dropwise add bromoethane 2.2mL and stir. The reaction solution gradually turned light gray. After 1.5 h, 2.5 mL of cyclopropyne was added and stirring was continued, and bubbles were released. After 1.5 hours, 0.75 g of ground polyoxymethylene was added to the reaction vessel and reacted overnight. The next day, 20 mL of 1 mol / L dilute hydrochloric acid was added to the system and the reaction solution was extracted (extractant: ethyl acetate, 30 mL×3). The extract was spin-dried and transferred to a 50mL flask, and a stirring bar and 1mL of water were added. Weigh 1 g of sodium hydroxide and dissolve it in 5 mL of water, transfer it to a flask, add 1.2 mL of dimethyl sulfate dropwise, and heat to re...
Embodiment 2
[0037] Use other bases to replace ethylmagnesium bromide, others are the same as Example 1, and the results are as follows image 3 The effect of different bases is shown. The results show that using n-butyllithium yield is slightly higher, but considering the convenience of operation and low cost, preferably ethylmagnesium bromide is used as alkali.
Embodiment 3
[0039] Use other methods to protect the hydroxyl group of cyclopropyl propargyl alcohol, others are the same as Example 1, and the results are as follows Figure 4 Effect of different protecting groups shown. The results show that the use of dimethyl sulfate protects the highest yield (Example 1), high atom economy and cheap reagents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com