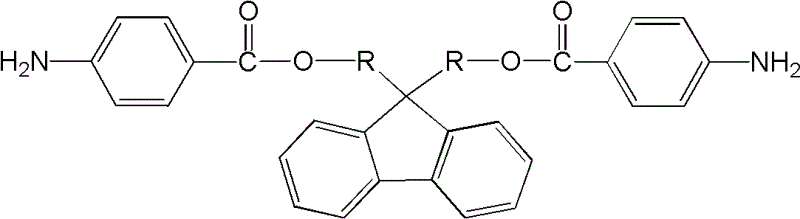
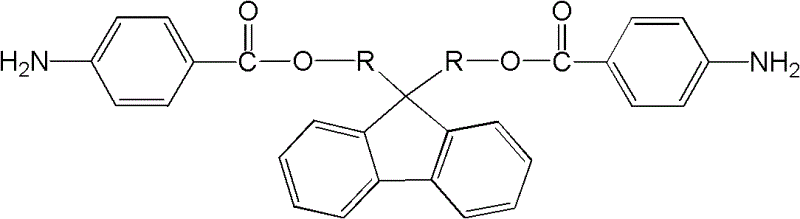
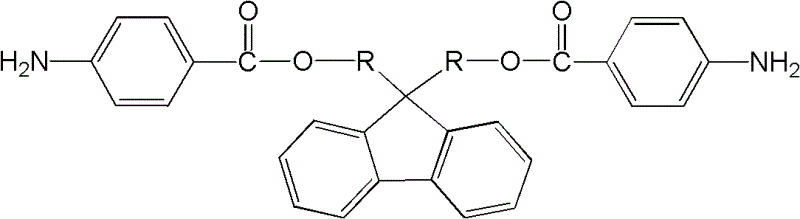
2-amino-fluorene containing ester group and preparation method thereof
A technology containing ester group-containing diaminofluorene and ester group-containing dinitrofluorene, which is applied in the field of organic polymer materials, can solve the problems of poor toughness of polymers and high rigidity of molecular chains, and achieve the effect of improving flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The basic preparation method of ester group-containing diaminofluorene of the present invention comprises:
[0019] The first step: Add dihydroxyfluorene, organic solvent and triethylamine in sequence to the container equipped with agitator, condenser and thermometer. The concentration of dihydroxyfluorene in the organic solvent is 50-200g / L, organic solvent and triethylamine The volume ratio of ethylamine is 1:1. Under the condition of -10~10℃, add dropwise to the organic solvent containing p-nitrobenzoyl chloride prepared in advance. The concentration of p-nitrobenzoyl chloride solution is 10~40g / L, the molar ratio of dihydroxyfluorene to p-nitrobenzoyl chloride is 1:2~2.2, after the dropwise addition, react at room temperature for 1~5 hours, heat to reflux, reaction time 10~24 hours, rotary evaporation Remove the organic solvent, then wash with water, methanol or ethanol, filter, and vacuum-dry to obtain dinitrofluorene containing ester groups;
[0020] The second ...
Embodiment 1
[0028] Synthesis of 9,9-bis(4-nitrobenzoic acid methyl)fluorene
[0029] Into a container equipped with a stirrer, a condenser, and a thermometer, add anhydrous chloroform 50mL, 9,9-bishydroxymethylfluorene 4.52g and triethylamine 50mL in sequence at 2 to 4°C, and then add dropwise at a concentration of 20g / L p-nitrobenzoyl chloride chloroform solution 375mL, after dropwise addition, react at room temperature for 3 hours, and at reflux temperature for 16 hours, after the reaction is over, distill off chloroform and triethylamine under reduced pressure, then wash with water, Wash with methanol or ethanol, filter, and vacuum-dry to obtain light yellow 9,9-bis(4-nitrobenzoic acid methyl)fluorene with a yield of 90%.
[0030] Proton NMR spectrum test result (500M, CDCl 3 , ppm): 7.35~7.84 (16H, Ar-H), 4.80 (4H, -CH 2 -). Infrared spectrum test results (KBr, cm -1): 2942 and 2833 are C-H stretching vibrations, 1740 shows typical carboxylate C=O stretching vibrations, 1601, 150...
Embodiment 2
[0032] Except the raw material 9,9-bishydroxymethyl fluorene was changed to 9,9-bis hydroxybutyl fluorene, the dosage was 6.2g, anhydrous chloroform was changed to dioxane, and the concentration of p-nitrobenzoyl chloride dioxane solution was It is 40g / L, and consumption is 190mL, other conditions are the same as embodiment 1, finally obtain light yellow 9,9-bis(4-nitrobenzoic acid butyl) fluorene, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com