Method for producing D-phenylalanine by using L-phenylalanine as raw material
A technology for phenylalanine and raw materials, which is applied in the field of D-enzyme splitting and preparing D-phenylalanine, can solve the problems of high cost, long process flow, low optical purity and the like, and achieves low cost, simple process flow and high product quality. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
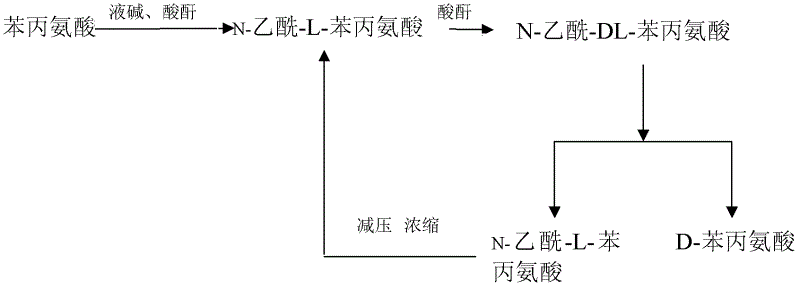
[0022] Acylation of L-phenylalanine
[0023] Weigh 300kgL-phenylalanine and dissolve it in 800L pure water, adjust the pH to 8-9 with 30% NaOH solution, about 200L of 30% NaOH is required. After the dissolution is complete, control the solution temperature at 30-35°C, and add acetic anhydride dropwise until the solution reacts with ninhydrin without color change, and the reaction is complete.
[0024] Acetyl-L-phenylalanine racemization
[0025] Use 30% hydrochloric acid to adjust the pH to 4-5 with about 0.8m3 acetylated-L-phenylalanine acylation solution with a concentration of about 0.04kg / L obtained in the previous step, add acetic anhydride dropwise at 60°C for racemization, Detection of racemate After the reaction, add 30% hydrochloric acid to adjust the pH to 1-2, cool and crystallize, filter out the crystals and dry them to obtain 30 kg of acetyl-DL-phenylalanine.
[0026] Resolution and separation of D-phenylalanine
[0027] Weigh 200kg of acetyl-DL-phenylalanine...
Embodiment 2
[0029] Acylation of L-phenylalanine
[0030] Weigh 330kgL-phenylalanine and dissolve it in 800L pure water, adjust the pH to 10-11 with 30% NaOH solution, about 200L of 30% NaOH is required. After the dissolution is complete, control the solution temperature at 35-40°C, and add acetic anhydride dropwise until the solution reacts with ninhydrin without color change, and the reaction is complete.
[0031] Acetyl-L-phenylalanine racemization
[0032] Use 30% hydrochloric acid to adjust the pH to 5-6 with about 0.8m3 acetylated-L-phenylalanine concentration of 0.04kg / L obtained in the previous step, add acetic anhydride dropwise at 70°C for racemization, and detect racemic solution After the reaction, add 30% hydrochloric acid to adjust the pH to 1-2, cool and crystallize, filter out the crystals and dry them to obtain 30 kg of acetyl-DL-phenylalanine.
[0033] Resolution and separation of D-phenylalanine
[0034] Weigh 200kg of acetyl-DL-phenylalanine to prepare a solution w...
Embodiment 3
[0036] Acylation of L-phenylalanine
[0037] Weigh 400kgL-phenylalanine and dissolve it in 800L pure water, and adjust the pH to 12-13 with 30% NaOH solution, and about 200L of 30% NaOH is required. After the dissolution is complete, control the solution temperature at 35-40°C, and add acetic anhydride dropwise until the solution reacts with ninhydrin without color change, and the reaction is complete.
[0038] Acetyl-L-phenylalanine racemization
[0039] Use 30% hydrochloric acid to adjust the pH to 5-6 with about 0.8m3 acetylated-L-phenylalanine concentration of 0.04kg / L obtained in the previous step, add acetic anhydride dropwise at 90°C for racemization, and detect racemic solution After the reaction, add 30% hydrochloric acid to adjust the pH to 3-4, cool and crystallize, filter out the crystals and dry them to obtain 30 kg of acetyl-DL-phenylalanine.
[0040] Resolution and separation of D-phenylalanine
[0041] Weigh 200kg of acetyl-DL-phenylalanine to prepare a so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com