Novel montmorillonite nanometer complex and preparation method thereof
A nanocomposite and montmorillonite technology, applied in chemical instruments and methods, silicon compounds, inorganic chemistry, etc., to achieve mild conditions, large specific surface area, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dissolve 5.6g of titanium tetrachloride in 100ml of dilute hydrochloric acid with a concentration of 1mol / L to prepare a titanium intercalation solution. 15g of sodium-based montmorillonite was dispersed in 750ml of deionized water to prepare a montmorillonite suspension. Under the conditions of room temperature and magnetic stirring, the titanium intercalation solution was slowly dropped into the montmorillonite suspension. After the dropwise addition, the reaction system was heated to 80°C and refluxed for 0.5 hours under magnetic stirring to obtain a titanium intercalation montmorillonite mixture. liquid. The reaction mixture was centrifuged to obtain titanium-montmorillonite.
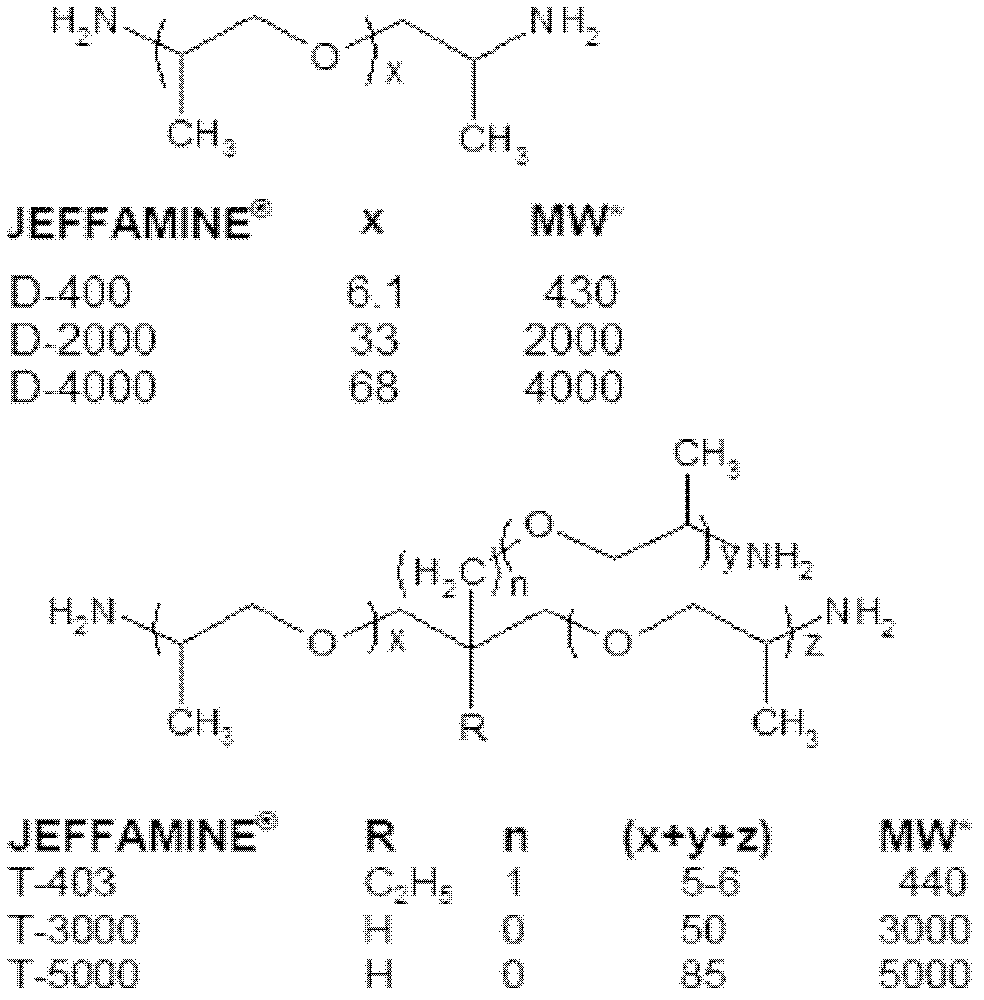
[0019] Dissolve 1.7g of polyetheramine D400 in 36ml of acetone, mix 1.5g of hydrochloric acid solution (37wt%) with 12ml of deionized water, add hydrochloric acid solution dropwise to D400 acetone solution under magnetic stirring, and continue magnetic stirring for 3h after the addition is c...
Embodiment 2
[0021] Dissolve 2.8g of titanium tetrachloride in 100ml of dilute hydrochloric acid with a concentration of 1mol / L to prepare a titanium intercalation solution. 15g of sodium-based montmorillonite was dispersed in 100ml of absolute ethanol to prepare a montmorillonite suspension. Under the conditions of room temperature and magnetic stirring, the titanium intercalation solution was slowly dropped into the montmorillonite suspension. After the dropwise addition, the reaction system was heated to 60°C and refluxed for 8 hours under magnetic stirring to obtain a titanium intercalation montmorillonite mixture. liquid. The reaction mixture was centrifuged to separate the titanium-intercalated montmorillonite.
[0022] 25g of polyetheramine D2000, 2.6g of hydrochloric acid solution (37wt%) and 20mL of deionized water were uniformly mixed, and then magnetically stirred for 3 hours to obtain D2000 polyether ammonium salt. Disperse the titanium intercalated montmorillonite in a mixed...
Embodiment 3
[0024] Dissolve 25g of polyetheramine T5000 in 48ml of acetone, add 1.5g of hydrochloric acid solution (37wt%) and 20mL of deionized water mixture under magnetic stirring at room temperature, and continue magnetic stirring for 3 hours to obtain T5000 polyether ammonium salt intercalation solution. Disperse 15g of sodium-based montmorillonite in 1000ml of acetone to prepare a montmorillonite suspension. Under the conditions of room temperature and magnetic stirring, the T5000 polyether ammonium salt intercalation solution was slowly dropped into the montmorillonite suspension. After the dropwise addition, the reaction system was heated to 70°C and refluxed for 4 hours under magnetic stirring to obtain T5000 Polyether ammonium salt intercalation montmorillonite mixture. The reaction mixture was centrifuged to separate the T5000 polyether ammonium salt intercalated montmorillonite.
[0025] Dissolve 0.07g of titanium tetrachloride in 100ml of dilute hydrochloric acid with a conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com