Nickel-base metal catalyst for preparing hydrogen by hydrazine decomposition at room temperature, as well as preparation and application thereof
A catalyst and base metal technology, applied in the field of nickel-based metal catalysts and their preparation, can solve the problems of low catalytic activity and slow hydrogen production rate, and achieve the effects of high catalytic activity and selectivity, easy separation and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of single-site catalysts
[0031] Weigh 11.77g nickel nitrate (Ni(NO 3 ) 3 ·6H 2 O) and 5.06g aluminum nitrate (Al(NO 3 ) 3 9H 2 O) was dissolved in 50mL deionized water to make solution A. Take by weighing 4.29g anhydrous sodium carbonate (Na 2 CO 3 ) was dissolved in 30 mL of deionized water and mixed with 20 mL of 3M NaOH solution to obtain solution B. Solution B was placed in a water bath at 35°C, and solution A was added to it at a rate of 3 mL / min under vigorous stirring, and a small amount of NaOH solution was added to adjust the pH to 10. The precipitate was crystallized in a 65°C water bath for 18h. After filtering and washing, the samples were dried at 80°C. XRD results confirmed that a hydrotalcite structure was formed, and its BET surface area was: 264.3m 2 / g. The above hydrotalcite precursor was heated at 500 °C in 10% H 2 / He atmosphere for 1 h to obtain a single-site catalyst.
Embodiment 2
[0033] Preparation of dual-site catalysts
[0034] 1 g of the nickel-aluminum hydrotalcite prepared in Example 1 was redispersed in 100 mL of deionized water to make a suspension, and stirred in a water bath at 65° C. for 4 h. Accurately measure the H required for a precious metal loading of 4wt% 2 IrCl 4 solution, H 2 PtCl 6 solution, RhCl 3The solution is slowly added dropwise to the above suspension, and 2.5-5g of urea is added as a precipitating agent. Then the temperature was raised to 90°C in a water bath and kept for 16h. After filtering and washing, the samples were dried at 80° C. to obtain a series of catalyst precursors loaded with precious metals. Weigh 0.1g of catalysts loaded with different precious metals, 2 / He reduction for 1 h to obtain a dual-site catalyst.
Embodiment 4
[0039] Catalyst Activity Test
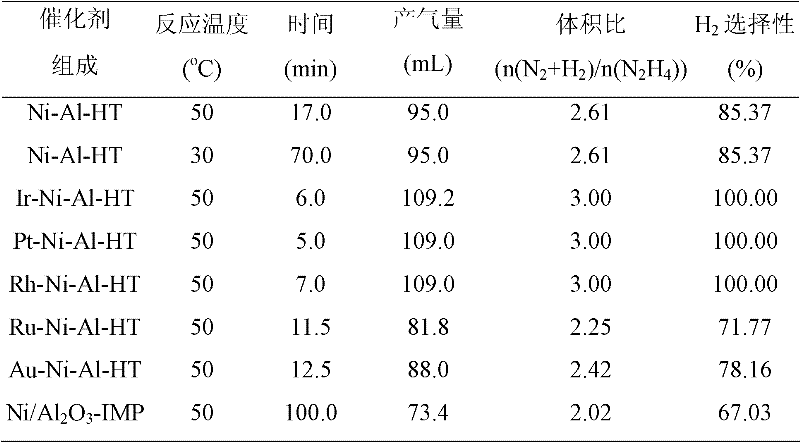
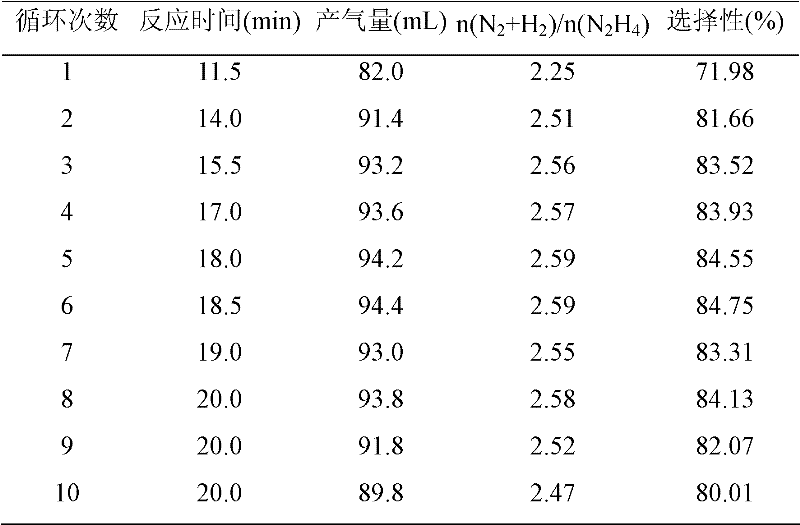
[0040] Catalyst evaluations of the present invention were performed in a closed drainage system. The experimental process is as follows: the reaction is carried out in a constant temperature water bath, and 4 mL of deionized water and catalyst are added to a three-necked flask. The catalyst prepared in embodiment 1 is Ni-Al-HT (HT: hydrotalcite), the catalyst prepared in embodiment 2 is X-Ni-Al-HT (X is Pt, Ir, Rh, Ru, Au), comparative example 3 The prepared catalyst is Ni-Al 2 o 3 -Imp. Then inject hydrazine hydrate in the above-mentioned three-necked bottle, and start timing simultaneously. The volume concentration of hydrazine hydrate used in the test is 13.65%. The gas generated by the catalytic decomposition of hydrazine is absorbed by the hydrochloric acid absorption device, and the rest is only hydrogen and nitrogen. The selectivity of the reaction can be calculated by reading the gas production. The activity test results of catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com