New method for preparing cytidine
A new method, the technology of cytidine, which is applied in the field of nucleoside preparation, can solve the problems that the preparation method of cytidine has not been reported in the literature, and achieve the effects of favorable production yield, good stability and stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
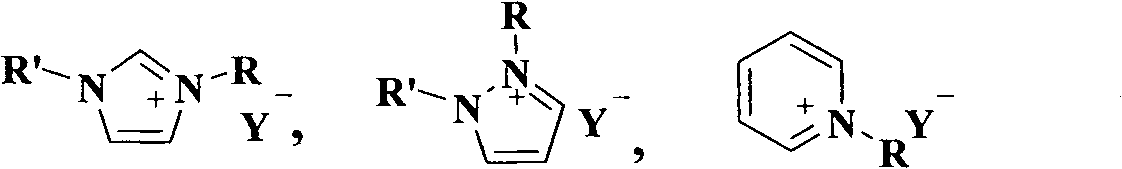
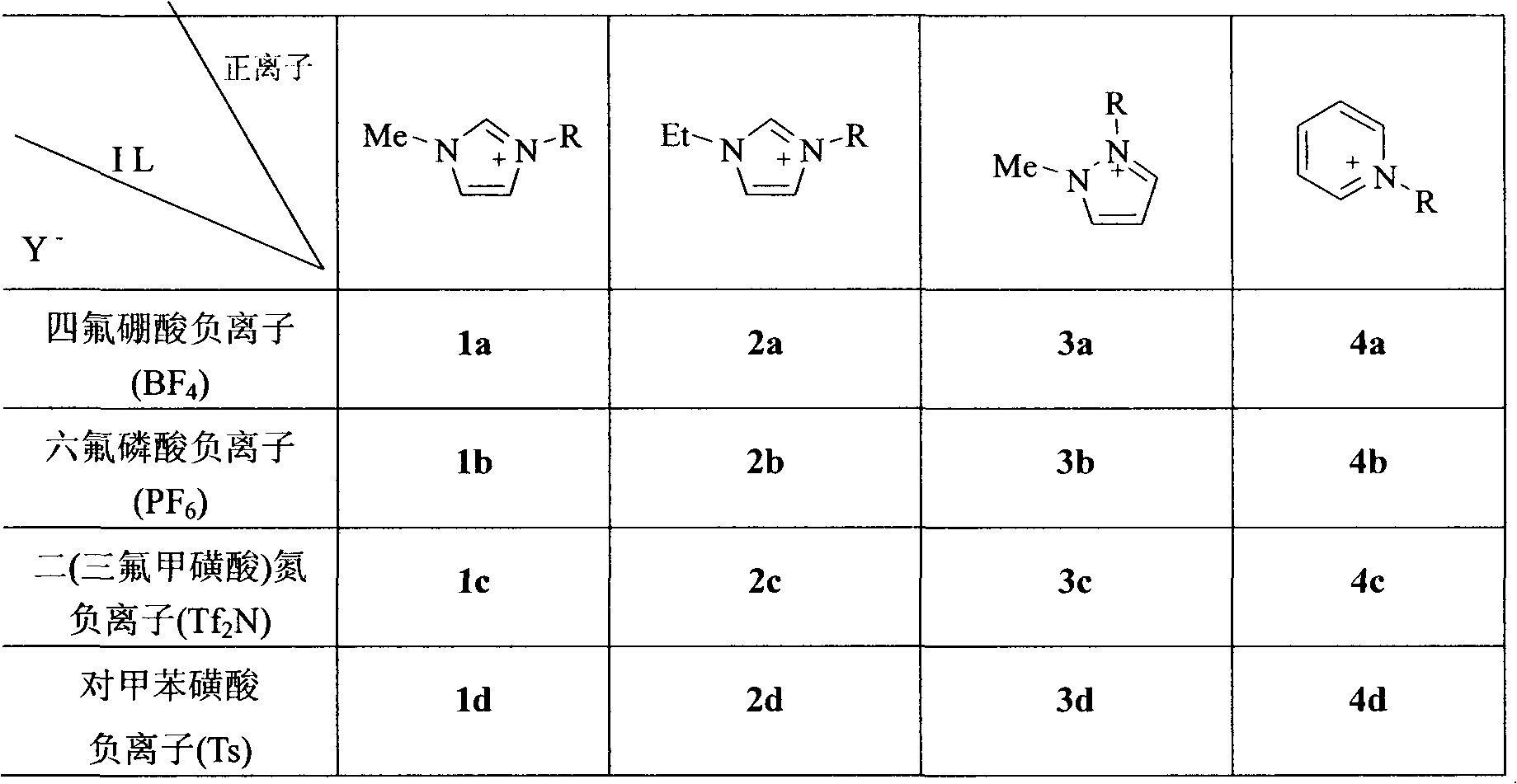
[0028] Acidic or partially acidic ionic liquid (Ionic Liquid, abbreviated as: IL) catalysts prepared a batch of combinations according to the following table 1 (the preparation method is referred to: Deng Youquan, Ionic Liquid---Properties, Preparation and Application, Beijing: China Petrochemical Press, 2006, p11-18) as a representative.
[0029] Table 1 Acidic or partially acidic ionic liquid catalysts
[0030]
[0031] Note: R in the table is n-butyl.
Embodiment 2
[0033] Preparation of cytidine
[0034]
[0035] 1.38g (4.33mmol) N 4 , O 2 -Dibenzoylcytosine and 1.53g (4.80mmol) tetraacetylribose were dissolved in 15mL of 1,2-dichloroethane, and the catalyst --- acidic ionic liquid 1a about 108mg (0.48mmol) was added at room temperature, and stirred 15h, thin plate chromatography detection, the reaction is complete. Add 20mL of ice water to the reaction solution, stir and separate the organic phase, then extract the water phase twice with 1,2-dichloroethane, combine the organic phases, dry over anhydrous sodium sulfate, concentrate and recover the solvent under negative pressure , to obtain a viscous intermediate. Then add 10 mL of 25% NH 3 - Methanol solution, react at 50-60°C for 4-5h, concentrate to dryness under negative pressure, add 5-7mL of methanol to dissolve the residue, and put the mixture in the refrigerator overnight. The resulting white solid precipitate was filtered, washed twice with a small amount of ice methanol...
Embodiment 3
[0039] Using the above raw material ratio and reaction conditions, and the operation method, the ionic liquid catalyst is changed, and the resulting cytidine yield is shown in the following table 2:
[0040] Cytidine productivity under different acidic ionic liquid catalyst conditions in table 2
[0041]
[0042] Note: "-" means not done.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com