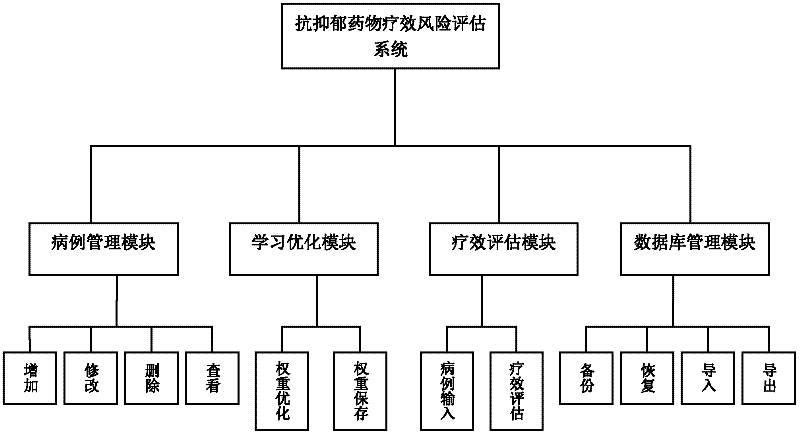
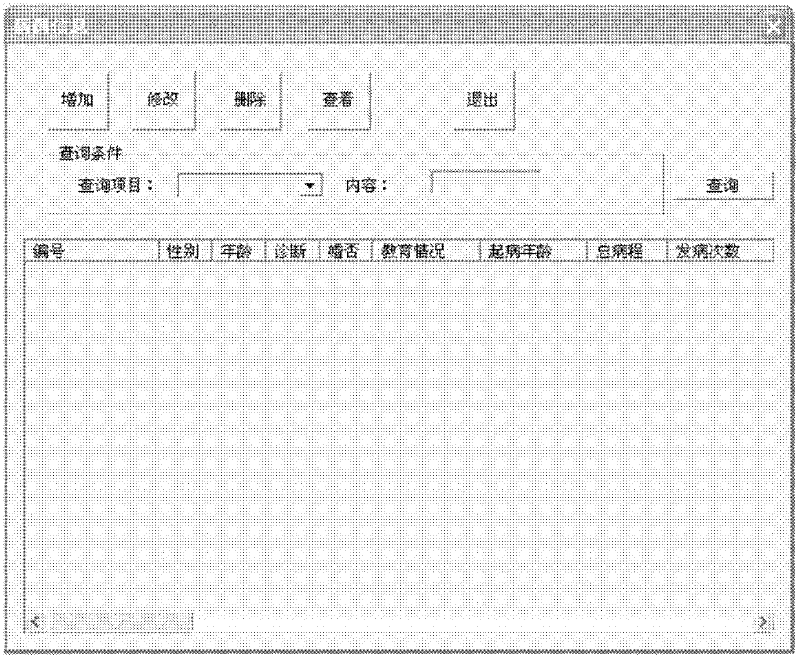
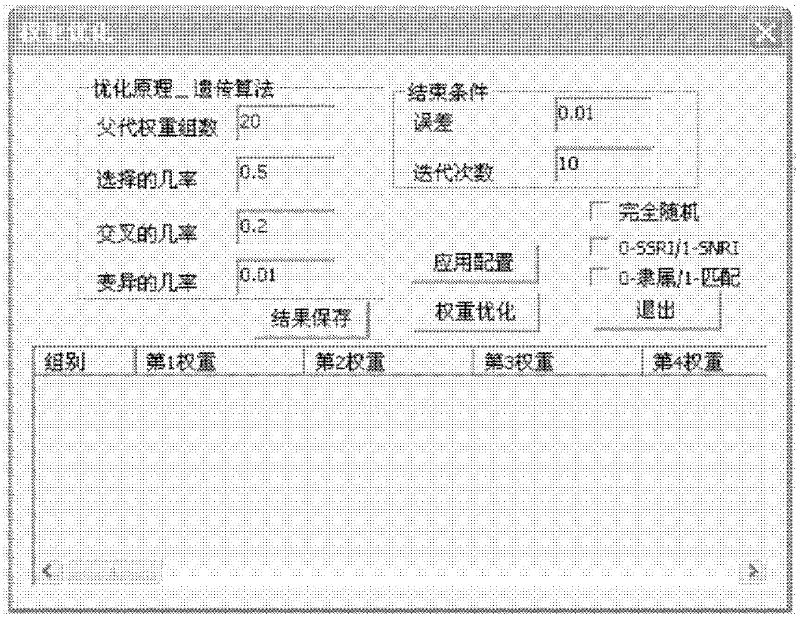
Biological chip-based antidepressant drug curative effect risk evaluating system and application thereof
A risk assessment system and biochip technology, which is applied in the field of antidepressant drug efficacy risk assessment system, can solve the problems that have not yet appeared in the field of antidepressant drug efficacy assessment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Enrollment and evaluation of clinical patients:
[0080] 1. Inclusion criteria:
[0081] Included in the group are Chinese Han depression patients who meet the diagnostic criteria of DSM-IV unipolar depression, Hamilton Depression Scale (HAMD-17) score ≥ 18, 18-60 years old, male or female, first-episode or relapse, and all of them signed the informed consent , approved by the Ethics Committee.
[0082] 2. Exclusion criteria:
[0083] Strictly exclude those with a history of schizophrenia, alcohol and drug dependence; those with a history of brain organic diseases and endocrine diseases; those with abnormal blood, heart, liver, and kidney functions after examination; pregnant and breastfeeding women; those with mania or hypomania Attack history; depressive episodes accompanied by psychotic symptoms; electroconvulsive therapy.
[0084] 3. Clinical evaluation:
[0085]3.1. Clinical data collection: name, gender, age, age of onset, frequency of onset, total course o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com