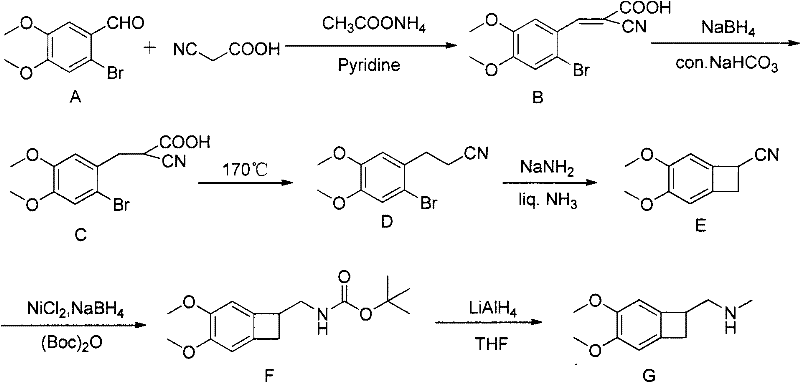
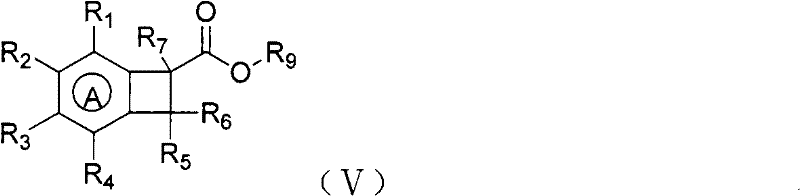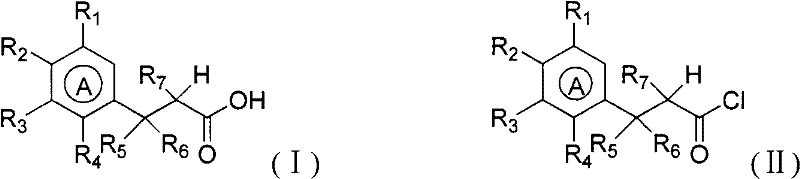Method for preparing benzocyclobutane compound and derivative thereof
A technology of benzocyclobutane and derivatives is applied in the field of preparation of benzocyclobutane compounds, and can solve the problems of harsh reaction conditions, low synthesis efficiency and high cost of synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of 3,4-dimethoxyphenylpropionyl chloride
[0051] Add 3,4-dimethoxyphenylpropionic acid (4g, 19.2mmol) in the single-neck round bottom flask of 100ml, add dichloromethane 15ml to dissolve, add thionyl chloride (4.2ml, 57.6mmol ), stirred at room temperature for 3 hours, and evaporated the solvent in the reaction solution in vacuo to obtain a brown-red oil.
Embodiment 2
[0052] Embodiment 2: the preparation of 5,6-dimethoxy-1-indanone
[0053] Add 3,4-dimethoxyphenylpropionyl chloride (19.2mmol) to a 100ml single-necked round-bottomed flask, add 30ml of dichloromethane to dissolve, and add AlCl to the reaction flask at room temperature 3 (5.1g, 38.4mmol), stirred at room temperature for 4 hours, after the reaction ended, the reaction solution was poured into ice water and stirred for 1 hour, extracted with dichloromethane (50ml×3 times), the organic layers were combined, and the organic layer was washed with water (50ml×1 times), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to obtain 3.62 g of khaki crystals with a yield of 98%. 1 H-NMR (300MHz, CDCl 3 ): δ7.18(s, 1H), δ6.89(s, 1H), δ3.97(s, 3H), δ3.91(s, 3H), δ3.05(t, 2H), δ2.68 (t, 2H).
Embodiment 3
[0054] Embodiment 3: the preparation of 2-bromo-5,6-dimethyl-indanone
[0055] The raw material 5,6-dimethoxy-1-indanone (3.62g, 18.9mmol), sodium bromide (3.89g, 37.8mmol) was added in a 100ml single-necked round bottom flask, 50ml of methanol was added, and the oil bath was heated to Reflux at 80°C for 8 hours, and the reaction was completed. After the reaction liquid was cooled to room temperature, it was suction filtered and concentrated to obtain 4.82 g of brown-yellow crystals. Yield 94%. 1 H-NMR (300MHz, CDCl 3 ): δ7.23(s, 1H), δ6.85(s, 1H), δ3.98(s, 3H), δ3.92(s, 3H), δ3.78(q, 1H), δ3.36 (q,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com