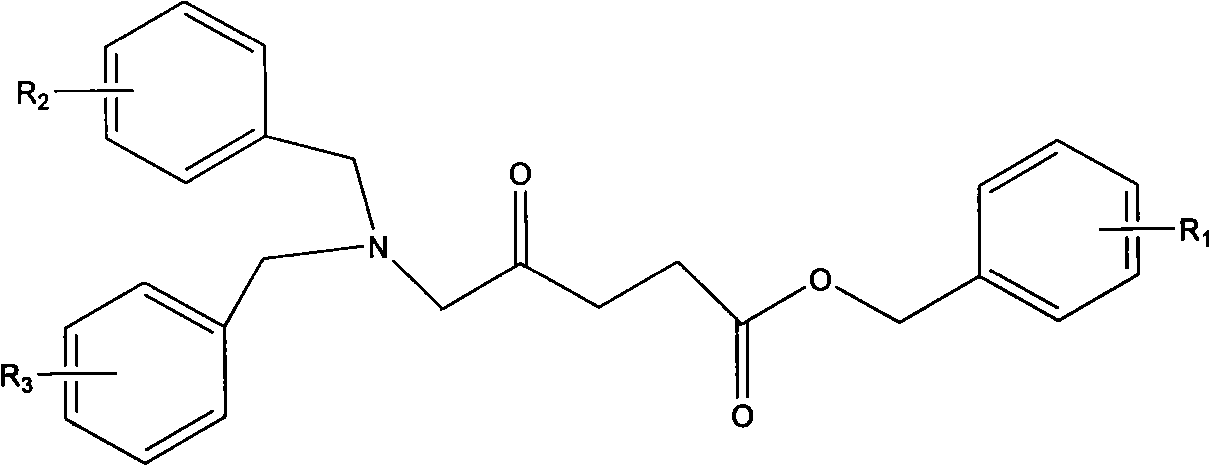
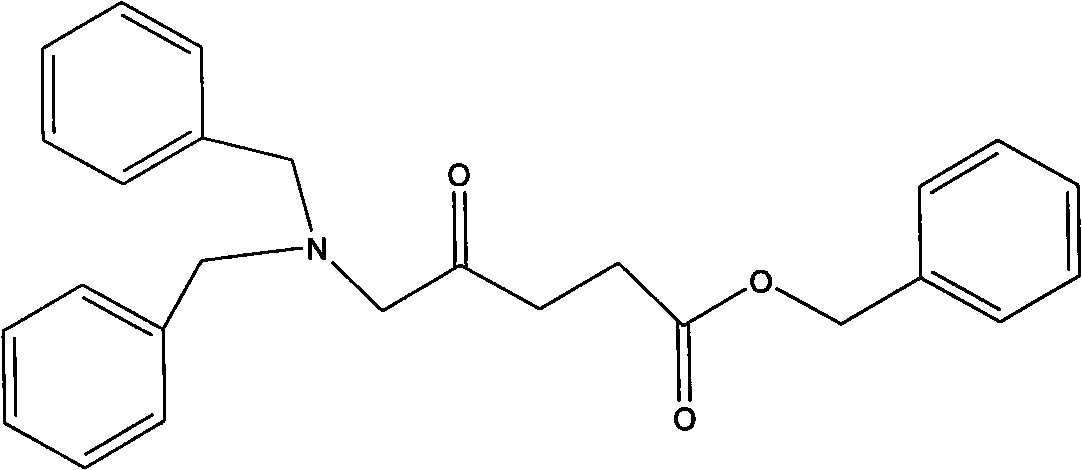
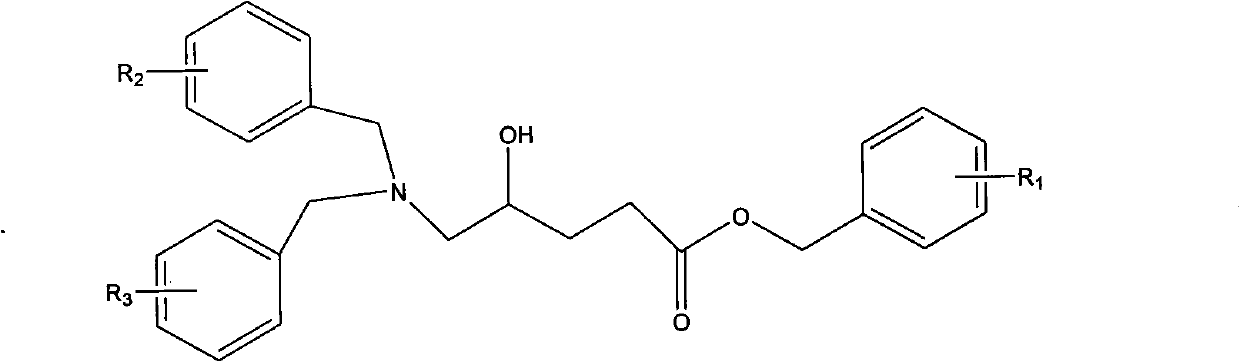
New preparation process of 5-aminolevulinic acid (5-ALA) hydrochloride
A new technology of aminolevulinic acid hydrochloride, which is applied in the field of new preparation technology of aminolevulinic acid hydrochloride, can solve the problems of difficult separation of intermediate products, low reaction yield, high cost of 5-ALA preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047] Preparation of epoxide 4-epoxyvalerate benzyl ester
[0048] In a 5000 milliliter flask, 155 grams of 4-alkene-pentanoic acid benzyl ester was dissolved in 1730 milliliters of dichloromethane, and 195 grams of m-chloroperoxybenzoic acid was added to maintain the reaction temperature from 30 to 35 degrees Celsius, and stirred for 24 hours. The reaction process A white solid precipitated out. TLC (developing solvent: cyclohexane-ethyl acetate 4:1) followed the reaction until the raw material 4-ene-pentanoic acid benzyl ester disappeared. Cool in a water bath, add 3% sulfurous acid solution, and test with potassium iodide starch test paper, the test paper does not show blue. Sodium bicarbonate solution was added to dissolve the solid, pH 7-8. Transfer to a separatory funnel and separate the layers. The organic matter was washed with 200 mL of brine. The organic layer was separated and dried over anhydrous sodium sulfate overnight. Suction filtration, concentration, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com