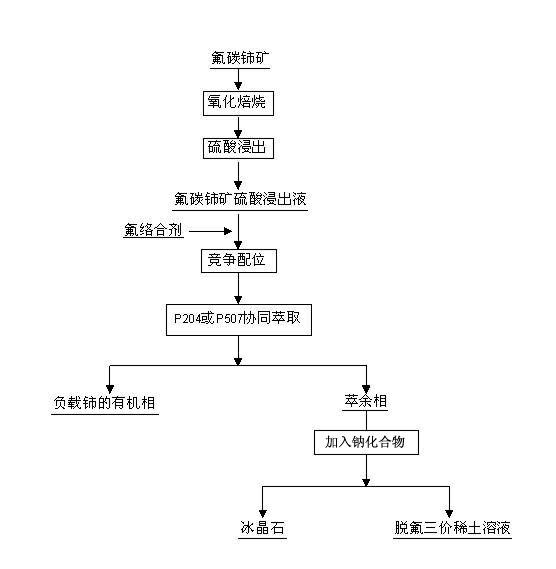
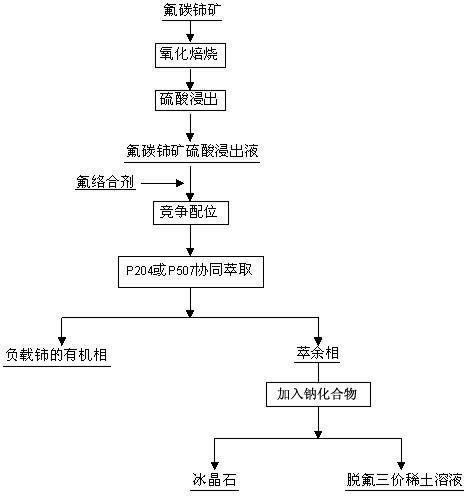
Method used for separating rare earth from bastnaesite sulphuric acid leach solution and preparing ice stone
A bastnasite and leaching solution technology, applied in aluminum fluoride, process efficiency improvement, aluminum halide and other directions, to achieve the effect of reducing recycling, reducing pollution and reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Bastnaesite was oxidized and roasted in the air at 400°C for 4 hours, and the concentration of bastnaesite was added to 1.0mol L -1 sulfuric acid, leached at 30°C for 4h, the liquid-solid weight ratio of sulfuric acid to bastnaesite was 5:1, to obtain bastnaesite sulfuric acid leaching solution, wherein Ce 4+ The concentration is 0.02 mol·L -1 , F - The concentration is 0.03mol·L -1 , RE 3+ The concentration is 0.35 mol·L -1 , the acidity of the solution is 0.2mol L -1 ;
[0029] (2) Add NaAlO to bastnaesite sulfuric acid leaching solution 2 , the molar ratio of fluorine to aluminum is 2:1, and the F in the sulfuric acid leaching solution - with NaAlO 2 Complexation, separation from cerium and trivalent rare earth elements;
[0030] (3) Mix P204 and sulfonated kerosene to obtain an organic phase, the concentration of the extractant is 0.1 mol / L, mix the organic phase with the above-mentioned bastnaesite sulfuric acid leaching solution at a volume ratio of 1:...
Embodiment 2
[0033] (1) The bastnaesite was oxidized and roasted in the air at 600°C for 2 hours, and the bastnaesite was added to the concentration of 0.5mol L after the oxidation roasting -1 Sulfuric acid, at 50 ℃ leaching time 2h, the liquid-solid weight ratio of sulfuric acid and bastnaesite is 10:1, obtain bastnaesite sulfuric acid leaching solution, wherein Ce 4+ The concentration is 0.015 mol·L -1 , F - The concentration is 0.03mol·L -1 , RE 3+ The concentration is 0.02mol·L -1 , the acidity of the solution is 1mol L -1 ;
[0034] (2) Add AlCl to the bastnaesite sulfuric acid leaching solution 3 , the molar ratio of fluorine to aluminum is 1:1, and the F in the sulfuric acid leaching solution - with AlCl 3 Complexation, separation from cerium and trivalent rare earth elements;
[0035] (3) Mix P507-P204 and sulfonated kerosene to obtain the organic phase, the mass fraction of P204 is 60%, the concentration of the extractant is 1mol / L, and the organic phase is mixed with the...
Embodiment 3
[0038] (1) Bastnaesite was oxidized and roasted in air at 800°C for 1 hour, and the concentration of bastnaesite was added to 2.0mol L after oxidation roasting -1 Sulfuric acid, leaching time 0.5h at 100°C, the liquid-solid weight ratio of sulfuric acid to bastnaesite is 1:1, to obtain bastnaesite sulfuric acid leaching solution, wherein Ce 4+ The concentration is 0.23mol L -1 , F - The concentration is 0.35mol L -1 , RE 3+ The concentration is 0.28 mol·L -1 ,, the acidity of the solution is 0.2mol L -1 ;
[0039] (2) Adding Al to bastnaesite sulfuric acid leaching solution 2 (SO 4 ) 3 , the molar ratio of fluorine to aluminum is 6:1, and the F in the sulfuric acid leaching solution - with Al 2 (SO 4 ) 3 Complexation, separation from cerium and trivalent rare earth elements;
[0040] (3) Mix P507 and sulfonated kerosene to obtain an organic phase, the concentration of the extractant is 3 mol / L, mix the organic phase with the above-mentioned bastnaesite sulfuric a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com