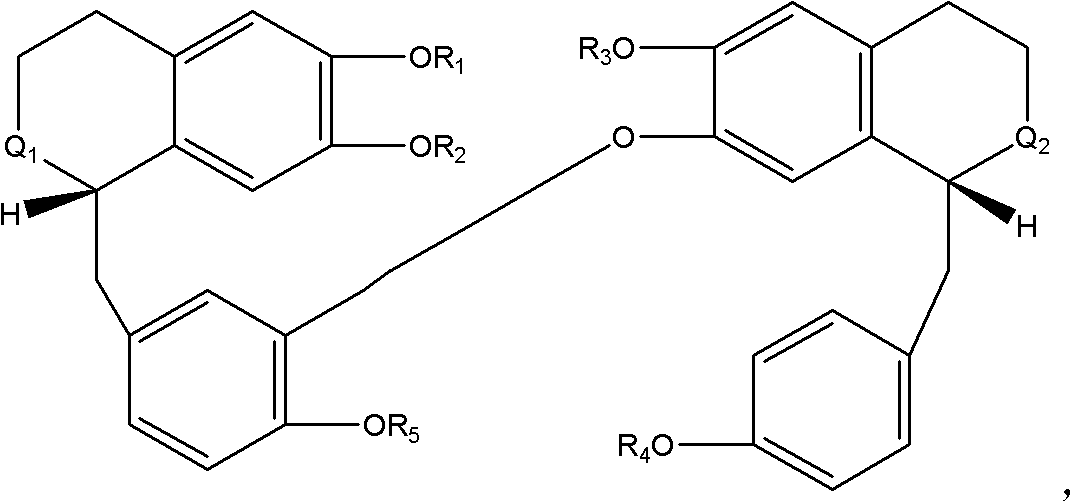
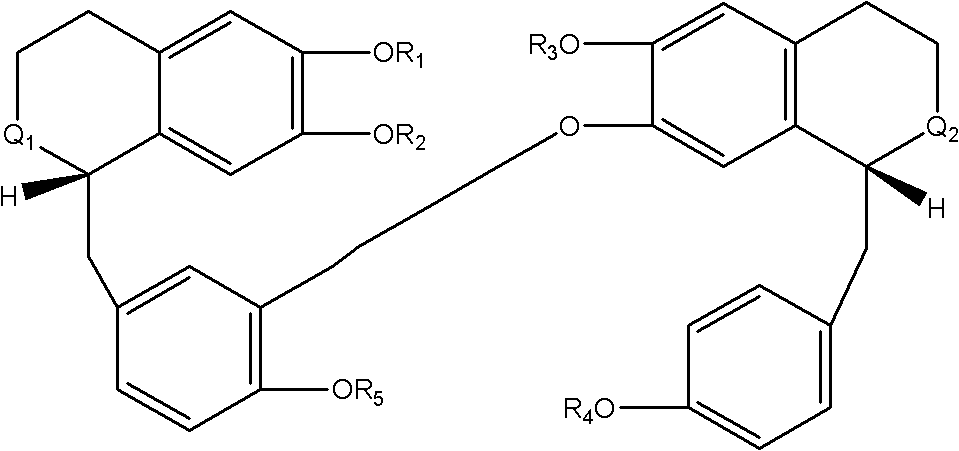
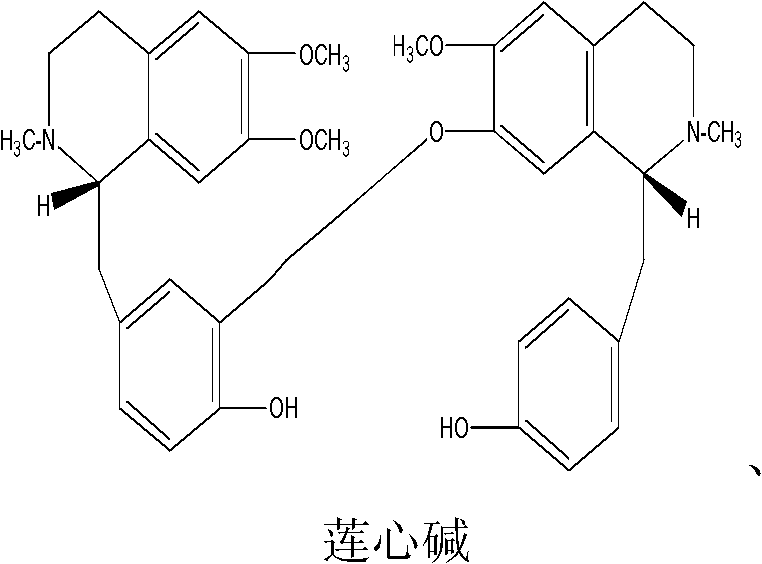
Novel uses of bisbenzylisoquinoline alkaloid derivative or analogue of general formula I
A technology of bisbenzylisoquinoline and alkaloid derivatives, which is applied in the field of medicine to achieve good inhibitory effect, reduce the thickening of the basement membrane, and restore the effect of the charge barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Clinical experiment of bisbenzylisoquinoline alkaloid derivatives of the present invention
[0033] 1.1 Test drug
[0034] Liensinine, isoliensinine, and neferine were separately packed into capsules, and placebo capsules (containing the same amount of starch) were made at the same time. The appearance and color of the capsules were consistent with those of Kaibotong capsules.
[0035] 1.2 Subject
[0036] A total of 100 patients with diabetic nephropathy (stage III or IV) entered the study and were randomly assigned to the liensinine group 30mg / Tid, isoliensinine group 30mg / Tid, neferine group 30mg / Tid, Kaibotong capsule group, and Placebo group / Tid. There was no significant difference in gender, age, course of disease and clinical symptoms in each group before entry (P>0.05), and they were comparable.
[0037] The patients in each group met the following conditions: (1) In line with diabetic nephropathy, according to Mogensen diabetic nephropathy stage III or IV, ur...
Embodiment 2
[0046] Example 2 Pharmacodynamic study of bisbenzylisoquinoline alkaloid derivatives of the present invention
[0047] 1Materials and methods
[0048] 1.1 Experimental materials
[0049] 1.1.1 Animals
[0050] Clean-grade healthy male Sprague-Dawley (SD) rats, weighing 180-200 g, were purchased from West China Experimental Animal Center of Sichuan University. In the environment of room temperature (22±1)℃, relative humidity (55±5)%, and photoperiod of 12h to 12h, they were adapted to rearing for 1wk and tested.
[0051] 1.1.2 Drugs
[0052] Liensinine, isoliensinine, neferine. Use normal saline to prepare gavage; Kaibotong (kaibotong) tablets, produced by Shanghai Zhongmei Squibb Pharmaceutical Co., Ltd., batch number: 1006041.
[0053] 1.1.3 Main reagents
[0054] Streptozotocin (STZ), Sigma company, dissolved in 0.01mol / L citrate buffer, pH 4.5 before use; rabbit anti-rat nephrin, podocin and HPSE polyclonal antibodies, Wuhan Boster; Biotin-labeled goat anti-rabbit IgG, horseradish pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com