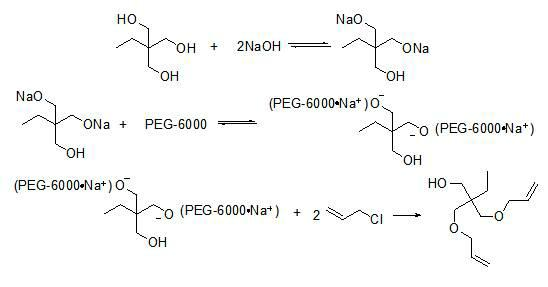
Method for synthesis of trimethylolpropane diallyl ether
A technology of methylolpropane diallyl ether and trimethylolpropane, which is applied in the field of synthesis of chemical intermediates, can solve the problems of incomplete utilization of raw materials, low product purity, unfavorable economic benefits, etc., and facilitates industrial production , The process conditions are not high, and the operation is simple and convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 80mL 1,4-dioxane, 134g (1 mol) trimethylolpropane, 7.16g PEG-6000 to a 500ml four-neck flask with a thermometer, condenser, constant pressure dropping funnel and stirrer (m PEG-6000 :m 三羟甲基丙烷 =0.05:1), start stirring, and heat up the water bath. After it is completely dissolved, add 160g of NaOH (4mol) crystals, the temperature continues to rise, and maintain the required reaction temperature in a water bath of 80°C. Increase the stirring intensity, then drop in 168.3g (2.2mol) propene chloride, and when propene chloride is added dropwise to nearly half, increase the rate of propene chloride addition. The total time for the dropwise addition was 4 hours. After the dropwise addition, the reaction was continued at 80° C. for 3 hours, the heating was stopped, and the stirring was turned off. The reacted solid-liquid mixture was cooled to room temperature, then filtered with a circulating water vacuum pump, and the filtrate was collected. Rotary evaporation was carri...
Embodiment 2
[0035] Add 80mL 1,4-dioxane, 134g (1 mol) trimethylolpropane, 4.07g PEG-6000 to a 500ml four-necked flask with a thermometer, condenser, constant pressure dropping funnel and stirrer (m PEG-6000 :m 三羟甲基丙烷 =0.03:1), start stirring, heat up the water bath, after it is completely dissolved, add 160g NaOH (4mol) crystals, the temperature continues to rise, and keep the temperature constant under the condition of 80°C water bath. Increase the stirring intensity, then drip 168.3g (2.2mol) propene chloride, and when propene chloride is added dropwise to nearly half, increase the rate of propene chloride addition. The total time for the dropwise addition was 4.5 hours. After the dropwise addition, the reaction was continued at 80° C. for 4 hours, the heating was stopped, and the stirring was turned off. The reacted solid-liquid mixture was cooled to room temperature, then filtered with a circulating water vacuum pump, and the filtrate was collected. Rotary evaporation was carried o...
Embodiment 3
[0037] Add 60mL 1,4-dioxane, 134g (1 mol) trimethylolpropane, 7.16g PEG-6000 to a 500ml four-necked flask with a thermometer, condenser, constant pressure dropping funnel and stirrer (m PEG-6000 :m 三羟甲基丙烷=0.05:1), start stirring, heat up the water bath, after it is completely dissolved, add 160g NaOH (4mol) crystals, the temperature continues to rise, and keep the temperature constant under the condition of 80°C water bath. Increase the stirring intensity, then drip 168.3g (2.2mol) propene chloride, and when propene chloride is added dropwise to nearly half, increase the rate of propene chloride addition. The total time for the dropwise addition was 4.5 hours. After the dropwise addition, the reaction was continued at 80° C. for 4 hours, the heating was stopped, and the stirring was turned off. The reacted solid-liquid mixture was cooled to room temperature, then filtered with a circulating water vacuum pump, and the filtrate was collected. Rotary evaporation was carried ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com