Naproxen eugenol ester medicinal compound and preparation method of naproxen eugenol ester medicinal compound
A technology of naproxen eugenol ester and eugenol ester, which is applied in the field of naproxen eugenol ester medicinal compound and its preparation, can solve problems such as eugenol irritation, and achieve reduced irritation, excellent analgesic activity, and gastrointestinal The effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
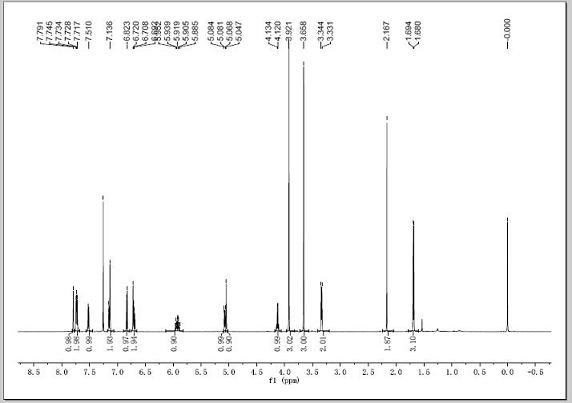
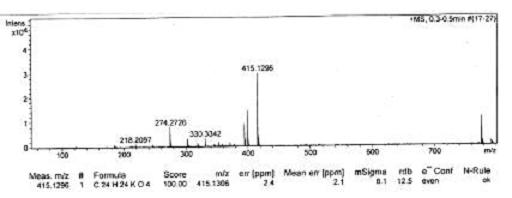
[0033] This example is the synthesis process of naproxen eugenol ester:
[0034] In a 250mL three-necked flask equipped with a stirrer and a thermometer, add 10g of naproxen and dissolve it in 80mL of dichloromethane, add 10mL of oxalyl chloride with an addition funnel, and react in an ice water bath at -5~0℃ for 3 hours. The excess oxalyl chloride is removed to obtain naproxenyl chloride.
[0035] Dissolve 5.5 g of eugenol in dichloromethane and 80 mL of the solution, add naproxen’s acid chloride with an addition funnel, react in an ice-water bath -5°C for 4 hours, filter, recover the dichloromethane, and dissolve the concentrated solution in dichloromethane. Wash once with saturated potassium carbonate solution and 3 times with distilled water. Dry the organic phase with anhydrous sodium sulfate overnight. Filter under reduced pressure and recover the solvent. Repeatedly recrystallize with ethyl acetate to obtain white solid crystals. The melting point is 120℃-121. ℃, that is, n...
Embodiment 2
[0037] This example is the synthesis process of naproxen eugenol ester:
[0038] In a 250mL three-necked flask equipped with a stirrer and a thermometer, add 12g of naproxen and dissolve it in 100mL of tetrahydrofuran, add 15mL of oxalyl chloride with an addition funnel, and react in an ice water bath at 0°C for 5 hours. The excess oxalyl chloride is evaporated under reduced pressure , Get naproxen acid chloride.
[0039] Dissolve 8.2 g of eugenol in 100 mL of tetrahydrofuran solution, add naproxen’s acid chloride with an addition funnel, react for 8 hours in an ice-water bath at 0°C, filter, and recover tetrahydrofuran. The concentrated solution is dissolved in dichloromethane and washed with saturated potassium carbonate solution. Once, washed with distilled water 3 times, the organic phase was dried overnight with anhydrous sodium sulfate, filtered under reduced pressure and the solvent was recovered, and recrystallized with ethyl acetate repeatedly to obtain white solid crystal...
Embodiment 3
[0041] This example is the synthesis process of naproxen eugenol ester:
[0042] In a 250mL three-necked flask equipped with a stirrer and a thermometer, add 11g of naproxen and dissolve it in 00mL of dichloromethane, add 12mL of oxalyl chloride with an addition funnel, and react in an ice water bath at -5℃ for 4 hours. Of oxalyl chloride to obtain naproxenyl chloride.
[0043] Dissolve 7 g of eugenol in 100 mL of dichloromethane solution, add naproxen’s acid chloride with an addition funnel, react for 6 hours in an ice-water bath at -5°C, filter, and recover dichloromethane. The concentrated solution is dissolved in dichloromethane and saturated Wash with potassium carbonate solution once, wash with distilled water 3 times, dry the organic phase with anhydrous sodium sulfate overnight, filter under reduced pressure and recover the solvent, recrystallize with ethyl acetate repeatedly to obtain white solid crystals, the melting point is 120℃-121℃, Namely naproxen eugenol ester....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com