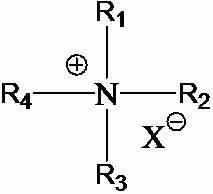
Quaternary ammonium class ionic liquid compound containing hydroxy groups and preparation method thereof
A technology for ionic liquids and compounds, which is applied in the field of quaternary ammonium ionic liquid compounds and their preparation, can solve the problems of inability to separate components and poor economic efficiency, and achieve the effects of green and environmental protection in the synthesis process, improved separation ability, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Mix 44.57 grams (0.5 moles) of N,N-dimethylethanolamine and 91.08 grams (0.5 moles) of triethyl phosphate in a round-bottomed flask with a condenser, and raise the temperature to 150°C through a constant temperature bath while stirring. The reaction was maintained at a constant temperature for 10 hours, and a weak nitrogen flow was maintained as a protective gas during the entire reaction process. After the reaction was completed and cooled to room temperature, the crude product was washed several times with ether, and then dried in vacuum at 120°C for 24 hours to obtain ethyl (hydroxyethyl) dimethyl quaternary ammonium diethyl phosphate. The reaction yield (in N , N-dimethylethanolamine) was 91.1%.
[0025] The result of the nuclear magnetic resonance analysis of this product is: 1 HNMR(300mHz, DMSO-d6): δ 3.97(2H); 3.57(4H); 3.43(2H); 3.30(6H); 3.28(2H); The analysis result of mass spectrometry (MS) was: m / z 271.2. Elemental analysis (EA) results were: C: 44.51%; H...
Embodiment 2
[0027] Mix 52.22 grams (0.35 moles) of triethanolamine and 133.16 grams (0.5 moles) of tributyl phosphate in a round-bottomed flask with a condenser, raise the temperature to 130 ° C through a constant temperature bath while stirring, and keep the constant temperature for 16 hours. A weak nitrogen flow was maintained as a protective gas throughout the reaction. After the reaction was completed and cooled to room temperature, the crude product was washed several times with ether, and then vacuum-dried at 120°C for 6 hours, that is, butyl trihydroxyethyl quaternary ammonium dibutyl phosphate, and the reaction yield (calculated as triethanolamine) was 93.6 %.
[0028] The result of the nuclear magnetic resonance analysis of this product is: 1 HNMR(300mHz, DMSO-d6): δ 3.97(6H); 3.53(6H); 3.24(2H); 1.73(2H); 1.48(4H); 1.33(6H); 0.96(9H). The analysis result of mass spectrometry (MS) was: m / z 415.5. Elemental analysis (EA) results were: C: 52.25%; H: 10.31%; N: 3.30% (calculated:...
Embodiment 3
[0030] Mix 143.45 grams (0.75 moles) of triisopropanolamine and 77.1 grams (0.5 moles) of diethyl sulfate in a round-bottomed flask with a condenser, raise the temperature to 100°C through a constant temperature bath while stirring, and maintain a constant temperature reaction For 24 hours, a slight nitrogen flow was maintained as a protective gas throughout the reaction. After the reaction was finished and cooled to room temperature, the crude product was washed several times with ethanol, and then vacuum-dried at 80°C for 48 hours to obtain trihydroxyisopropyl ethyl quaternary ammonium ethyl sulfate. The reaction yield (in terms of isopropanolamine ) was 80.3%.
[0031] The result of the nuclear magnetic resonance analysis of this product is: 1 HNMR(300mHz, DMSO-d6): δ 4.02(3H); 3.57(2H); 3.39(6H); 3.28(2H); 1.39(3H); 1.25(3H); 1.21(9H). The analysis result of mass spectrometry (MS) was: m / z 345.5. Elemental analysis (EA) results were: C: 45.02%; H: 8.81%; N: 4.11% (calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com