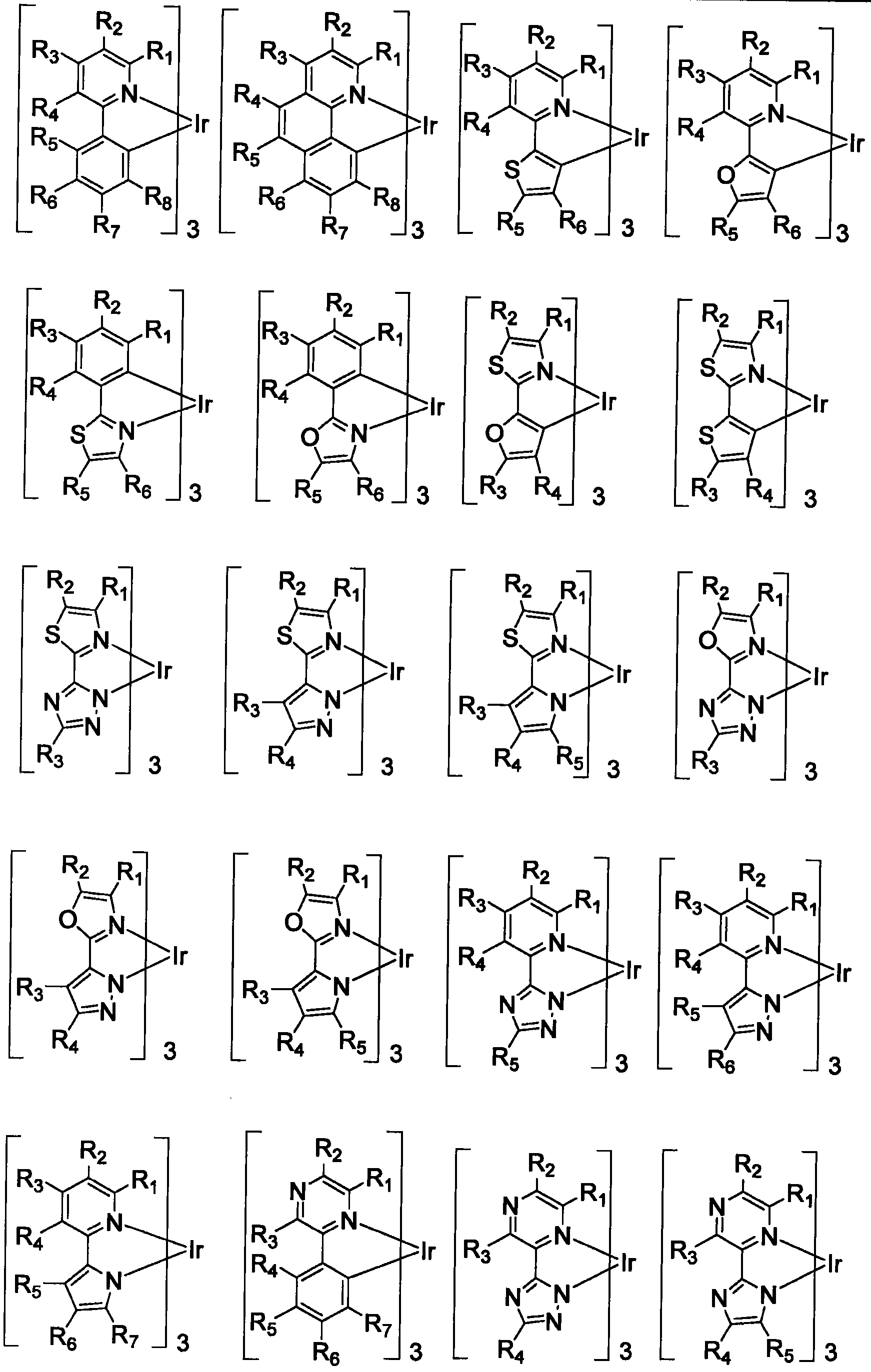
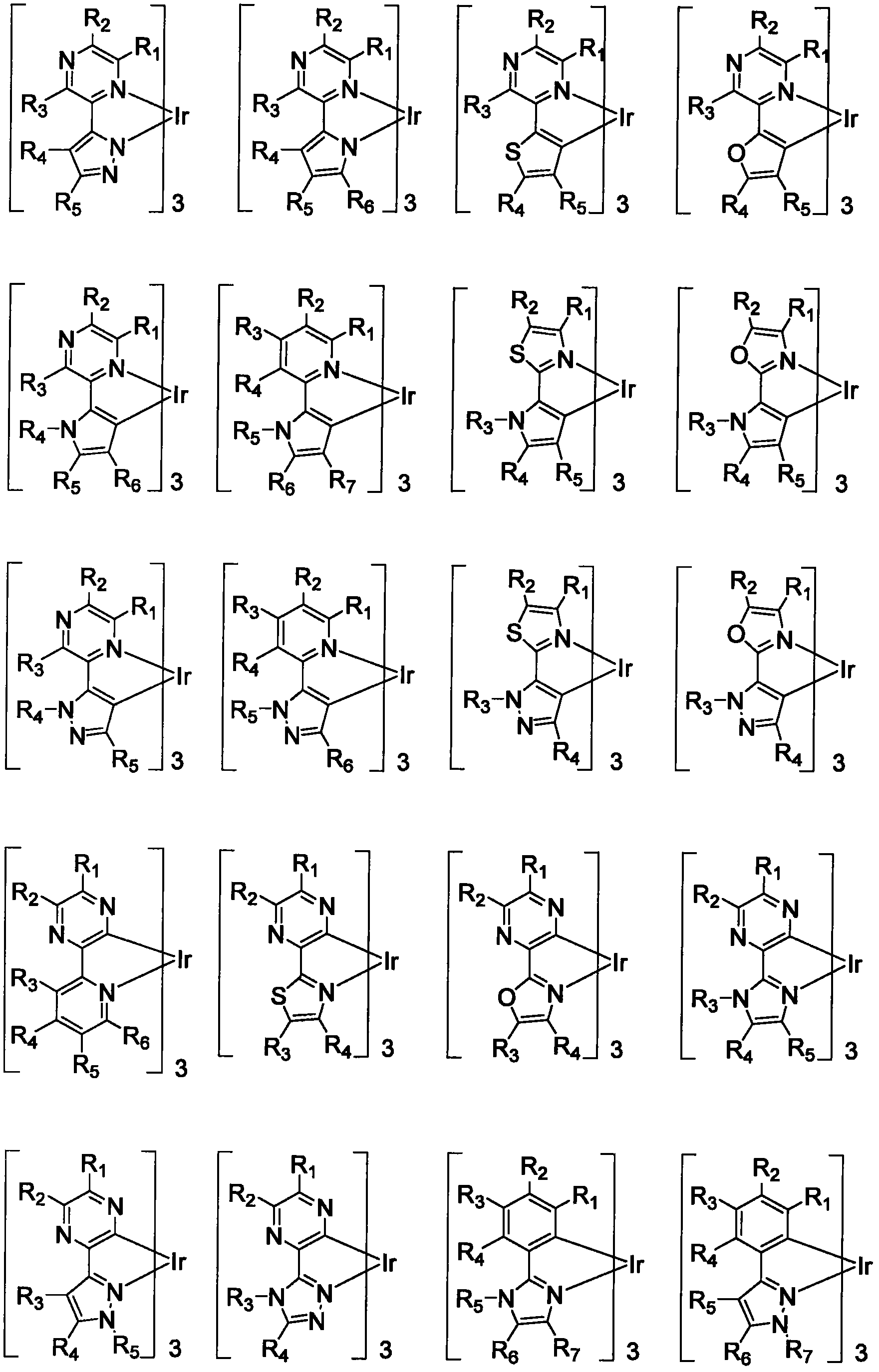
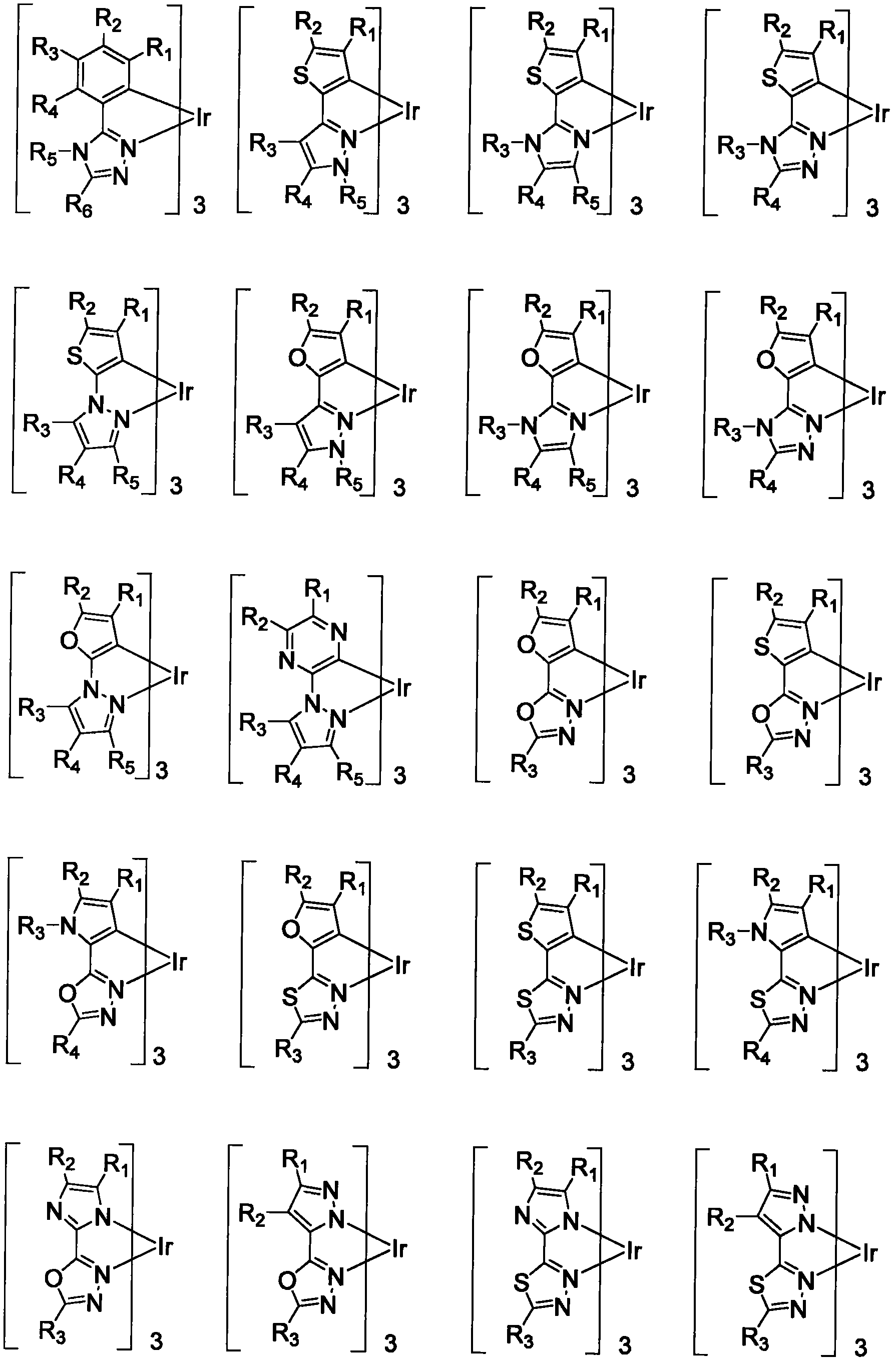
Functionalized triplet emitters for electro-luminescent devices
A ligand and negative charge technology, applied in electroluminescence light source, electric solid state device, electric light source, etc., can solve problems such as material stability and adverse effects on device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109]
[0110]
[0111] Bromofunctionalized phenylpyridines were synthesized at 200 °C with Ir(acac) 3 Complexation was carried out to ensure access to the facial isomer. Substituted emitters were synthesized by direct coupling of fac-iridium(III) complexes to triarylamine-boronate esters using Suzuki coupling.
[0112] Synthesis of Example 1
[0113] μ-Dichlorotetrakis(2-(3-bromophenyl-3-bromopyridinato)-κN, C)diiridium (97mg, 0.086mmol) and diphenyl-[4-[4,4, 5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl]amine (239 mg, 0,65 mol) and sodium carbonate (137 mg, 1,29 mmol) were added to distilled toluene (50 mL) , absolute ethanol (20 mL) and distilled water (15 mL). Before tetrakis(triphenylphosphine) palladium (30 mg, 0.026 mmol) was added, the white suspension was degassed for half an hour. The yellow two-phase mixture was Heat to 80 °C and stir overnight under nitrogen. The mixture is cooled to room temperature. The organic phase is separated and the aqueous phase is ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com