Method for separating phenylsuccinic acid enantiomer by extraction from multi-stage centrifugal inclusion reaction
A technology for phenylsuccinic acid and enantiomers is applied in the field of extraction and separation of enantiomers of phenylsuccinic acid by countercurrent inclusion reaction of a multistage centrifugal extractor, and can solve the problems of excess enantiomer and low yield, etc. To achieve the effect of stable product quality, easy operation and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 0.7687g of phenylsuccinic acid enantiomer was dissolved in n-octanol organic solvent to form a 4L solution with a concentration of 1mM as the material liquid phase, and pure n-octanol organic solvent was used as the organic phase.
[0013] Take 57.6400g water-soluble hydroxypropyl-β-cyclodextrin and dissolve it in 0.1M NaH 2 PO 4 / H 3 PO 4 Adjust the pH of the buffer solution to 3.0 to prepare 4L of aqueous solution with a concentration of 0.01M as the extraction phase.
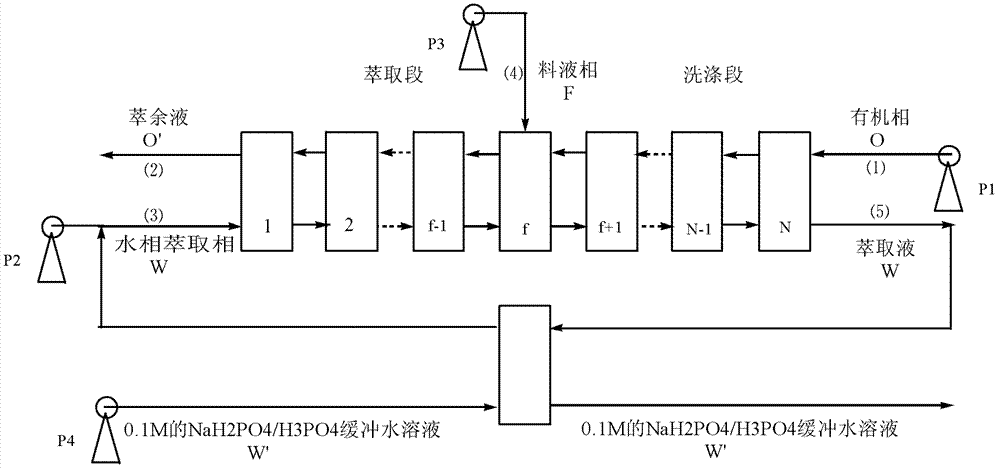
[0014] 8-stage centrifuges in series, using a centrifugal pump to pump the organic phase into the centrifuge first, when the organic phase flows out of the outlet, pump the aqueous phase extraction phase into the centrifuge from the corresponding inlet, after a period of time reaches equilibrium and then the liquid The phase is pumped in from the 4th stage centrifuge. The volume ratio of aqueous phase extraction phase: organic phase is 0.8:1, and the volume ratio of aqueous phase extraction phase: ...
Embodiment 2
[0016] 0.7687g phenylsuccinic acid enantiomer was dissolved in 1.2-dichloroethane organic solvent to prepare 4L solution with a concentration of 1mM as the material liquid phase, and pure 1.2-dichloroethane organic solvent as the organic phase.
[0017] Take 57.6400g water-soluble hydroxypropyl-β-cyclodextrin and dissolve it in 0.1M NaH 2 PO 4 / H 3 PO 4 Adjust the pH of the buffer solution to 3.0 to prepare 4L of aqueous solution with a concentration of 0.01M as the extraction phase.
[0018] 8-stage centrifuges in series, using a centrifugal pump to pump the organic phase into the centrifuge first, when the organic phase flows out of the outlet, pump the aqueous phase extraction phase into the centrifuge from the corresponding inlet, after a period of time reaches equilibrium and then the liquid The phase is pumped in from the 4th stage centrifuge. The volume ratio of aqueous extraction phase: organic phase is 1.5:1, and the volume ratio of aqueous extraction phase: solid...
Embodiment 3
[0020] Take 1.5374g of phenylsuccinic acid enantiomers and dissolve them in n-octanol organic solvent to form a 4L solution with a concentration of 2mM as the material liquid phase, and use pure n-octanol organic solvent as the organic phase.
[0021] Take 115.2800g water-soluble hydroxypropyl-β-cyclodextrin and dissolve it in 0.1M NaH 2 PO 4 / H 3 PO 4 Adjust the pH of the buffer solution to 2.5 to prepare 4L aqueous solution with a concentration of 0.02M as the extraction phase.
[0022] Connect 20-stage centrifuges in series, use a centrifugal pump to pump the organic phase into the centrifuge first, when the organic phase flows out from the outlet end, pump the aqueous phase extraction phase into the centrifuge from the corresponding inlet, after a period of time reaches equilibrium and then the liquid The phase is pumped in from the 10th stage centrifuge. The volume ratio of aqueous extraction phase: organic phase is 0.5:1, and the volume ratio of aqueous extraction ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com