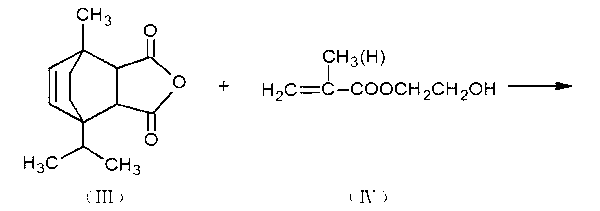Method for synthesizing tackifier monomer by utilizing terpinene serving as byproduct of terpinol
A technology of terpinene and tackifier, which is applied in the field of synthesizing tackifier monomers by using terpineol as a by-product of terpinene, which can solve problems such as difficult separation and unacceptable cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
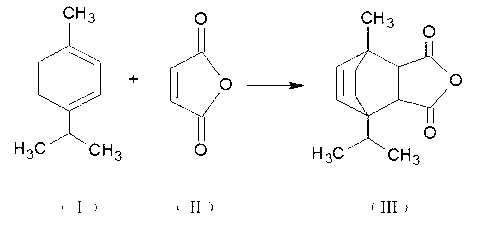
[0016] Take 125 grams of maleic anhydride and add it to a four-necked flask equipped with a stirring device, a reflux condenser, a dropping funnel and a thermometer, and heat up to raise the temperature. After the maleic anhydride is melted, start stirring and add 7 grams of catalyst, and raise the temperature to about 130°C , add 300 grams of terpinene dropwise, finish adding within 1.5-2 hours, raise the temperature to 140°C-150°C, react for 2-4 hours, cool down to room temperature in a water bath, add toluene to dilute the reactant, remove the catalyst by filtration, and distill out under reduced pressure Organic solvent and unreacted terpinene, obtain intermediate product;
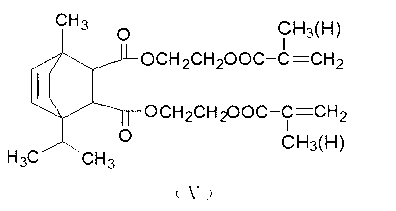
[0017] Take 200 grams of the intermediate product obtained from the above reaction and 0.56 grams of hydroquinone, and add them to a four-necked bottle equipped with a stirring device, a reflux condenser, a dropping funnel and a thermometer, slowly raise the temperature to about 160 ° C, start stirring,...
Embodiment 2
[0019] Take 100 grams of maleic anhydride and add it to a four-necked flask equipped with a stirring device, a reflux condenser, a dropping funnel and a thermometer, and heat up to raise the temperature. After the maleic anhydride is melted, start stirring and add 5 grams of catalyst, and raise the temperature to about 130°C , add 260 grams of terpinene dropwise, finish adding within 1.5-2 hours, raise the temperature to 140°C-150°C, react for 2-4 hours, cool down to room temperature in a water bath, add toluene to dilute the reactant, filter to remove the catalyst, and distill it under reduced pressure Organic solvent and unreacted terpinene, obtain intermediate product;
[0020] Take 180 grams of the intermediate product and 0.6 gram of hydroquinone obtained from the above reaction, and add them to a four-necked bottle equipped with a stirring device, a reflux condenser, a dropping funnel and a thermometer, slowly raise the temperature to about 160°C, start stirring, and star...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com