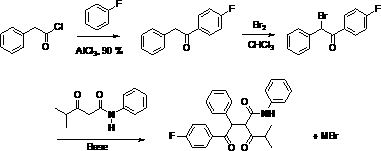
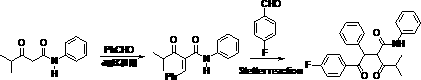
Method for preparing atorvastatin calcium intermediate
A technology for atorvastatin calcium and intermediates, which is applied in the field of preparation of atorvastatin calcium intermediates, and can solve the problems of long reaction steps, foul smell, and difficulty in obtaining catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
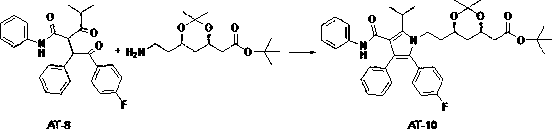
[0022] Dissolve Compound A (2.3g) and Compound B (2.7g) in 50ml of dry toluene solvent, heat to 45°C, stir for 30min, slowly add 3.5g of concentrated sulfuric acid dropwise, continue stirring for 30min, heat up to 90 ℃, the water separator separates the water. React for 24 hours. After the reaction, wash with water until neutral, and wash the organic phase with saturated sodium bicarbonate solution. Dry over anhydrous sodium sulfate. After evaporating the solvent under reduced pressure, dissolve it with 50ml ethyl acetate and stir it mechanically. Heat to 30°C, slowly add 150ml of petroleum ether dropwise to obtain the product. Repeat the above recrystallization steps twice to obtain 3.2g of the product.
Embodiment 2
[0024] Dissolve compound A (30g) and compound B (35g) in 300ml of dry toluene solvent, heat to 45°C, stir for 30min, slowly add 50g of concentrated phosphoric acid dropwise, continue stirring for 30min, raise the temperature to 90°C, separate the water The device divides the water. React for 26 hours. After the reaction, wash with water until neutral, and wash the organic phase with saturated sodium bicarbonate solution. Dry over anhydrous sodium sulfate. After distilling off the solvent under reduced pressure, dissolve it with 200ml of ethyl acetate and stir it mechanically. Heat to 30°C, slowly add 600ml of petroleum ether dropwise to obtain the product. Repeat the above recrystallization steps twice to obtain 44.8g of the product .
Embodiment 3
[0026] Dissolve compound A (100g) and compound B (115g) in 1000ml of dry toluene solvent, heat to 45°C, stir for 30min, slowly add 160g of concentrated phosphoric acid dropwise, continue stirring for 30min, heat up to 90°C, separate water Separate the water. React for 26 hours. After the reaction was completed, it was washed with water until neutral, and the organic phase was washed with saturated sodium bicarbonate solution. Dry over anhydrous sodium sulfate. After the solvent was evaporated under reduced pressure, it was dissolved in 1000ml ethyl acetate and stirred mechanically. Heat to 30°C, slowly add 3000ml of petroleum ether dropwise to obtain the product. Repeat the above recrystallization steps twice to obtain 151.0g of the product.
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com