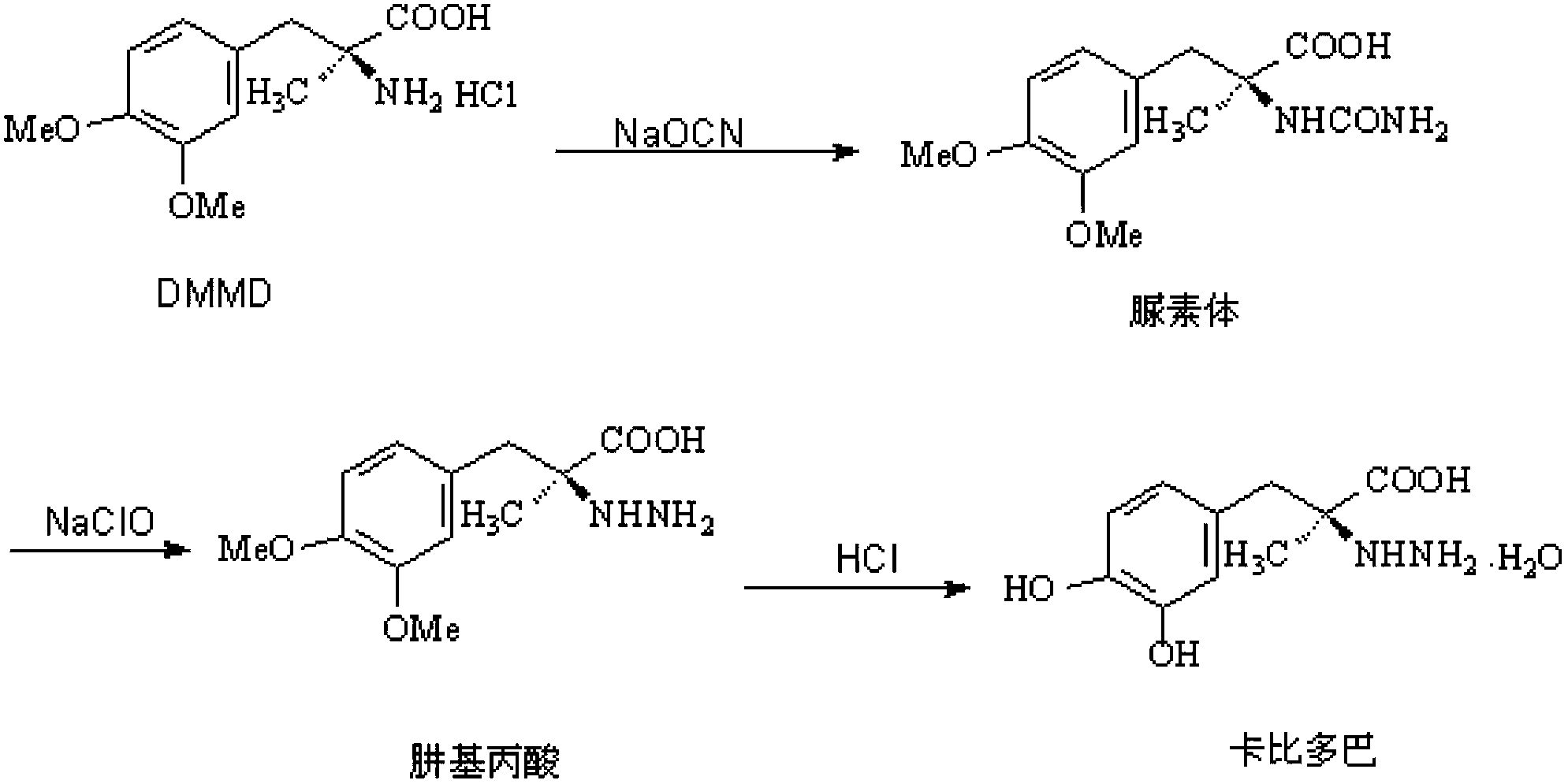
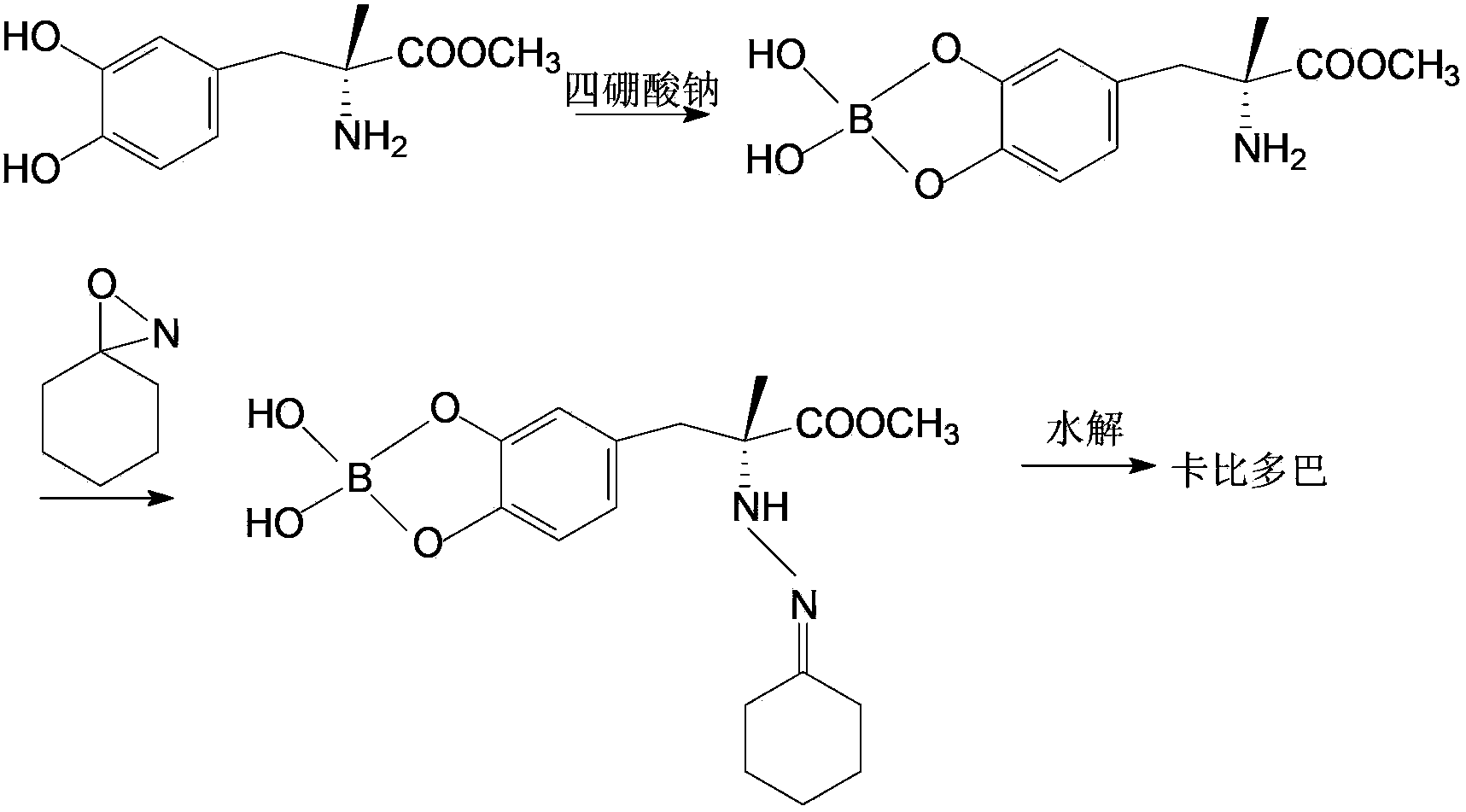
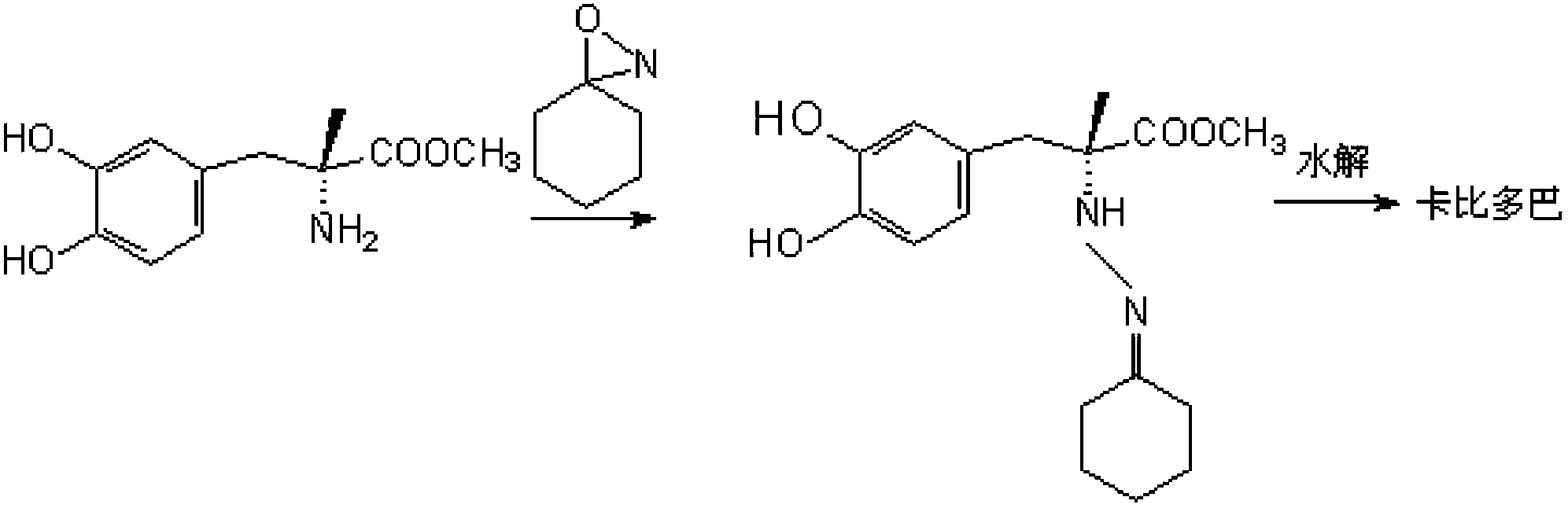
Method for synthesizing carbidopa
A synthetic method, the technology of oxyaziridine, applied in hydrazine preparation, organic chemistry, etc., can solve the problems of unsuitable carcinogenic effect of cyclohexanone, unqualified product residue, inconvenient recovery and application, etc., and achieve good product quality and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of Oxyaziridine
[0030] Add 0.044mol chloramine to 100ml of 1N NaOH solution under stirring to form a solution, then drop it into 100ml of dichloromethane solution containing 10ml of acetone under vigorous stirring, and control the temperature at 20°C. After adding, stir for 30 seconds and stop Stir and remove the aqueous layer. The dichloromethane layer contained 3,3-dimethyloxyaziridine in a theoretical amount of 43.5%. Dry the organic layer with anhydrous sodium sulfate, control the reflux ratio of 1:3 and concentrate with a fractionation column until the content of oxyaziridine is 0.9 mol / L.
[0031] (2) Preparation of imidate
[0032] With the dichloromethane solution of 0.073mol above-mentioned oxaziridine, control temperature is 25 ℃, drop in the 50ml dichloromethane liquid of the methyl dopa methyl ester of 0.068mol in 15~20 minutes, dripping finishes, in Stir at this temperature, cool down to 0°C, and filter to obtain methyl dopamine ester. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com